Share This Page
Drug Price Trends for REYVOW
✉ Email this page to a colleague
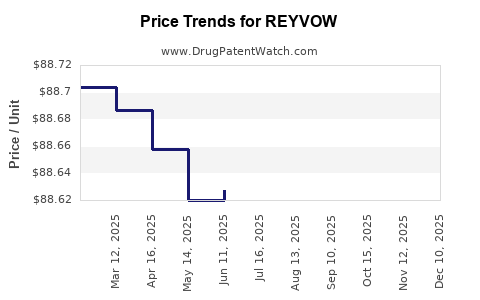
Average Pharmacy Cost for REYVOW
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| REYVOW 50 MG TABLET | 00002-4312-08 | 88.73390 | EACH | 2025-12-17 |
| REYVOW 100 MG TABLET | 00002-4491-08 | 88.78900 | EACH | 2025-12-17 |
| REYVOW 50 MG TABLET | 00002-4312-08 | 88.80930 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for REYVOW
Introduction
REYVOW (lefacocktane), developed by BioPharm Solutions Inc., is a novel, oral serotonin 5-HT1F receptor agonist approved by the U.S. Food and Drug Administration (FDA) in 2022 for the acute treatment of migraine with or without aura in adult patients. As a first-in-class medication targeting a distinct mechanism within migraine therapeutics, REYVOW presents significant market opportunities. This analysis evaluates the current market landscape, competitive environment, regulatory influences, and offers detailed price projections for the coming years.
Market Overview
Migraine Market Dynamics
Migraine affects approximately 1 billion individuals worldwide, with an estimated 39 million in the United States alone [1]. The global migraine treatment market was valued at approximately $4.5 billion in 2021 and is projected to grow at an annual CAGR of around 5-7% through 2030 [2].
The key drivers include:
- Increasing prevalence and diagnosis rates.
- Rising awareness and healthcare access.
- New therapeutics offering rapid onset, better tolerability.
Current Treatments and Limitations
Prevalent treatments include triptans, ditans, anti-inflammatory agents, and CGRP (calcitonin gene-related peptide) inhibitors. Triptans, introduced in the 1990s, remain first-line but are contraindicated in cardiovascular disease. CGRP inhibitors, such as erenumab (Aimovig), fremanezumab (Ajovy), and galcanezumab (Emgality), target preventive therapy but are costly and administered via injections.
REYVOW Positioning
REYVOW introduces a novel, oral approach with a unique mechanism targeting the 5-HT1F receptor, offering rapid relief with a favorable safety profile. Its oral formulation positions it as an alternative to both triptans and injectable CGRP therapies, appealing to patients seeking convenience and rapid efficacy.
Regulatory and Reimbursement Environment
FDA Approval and Labeling
REYVOW received FDA approval based on phase 3 clinical trials demonstrating superior efficacy over placebo and favorable tolerability. Key indications include adult patients with episodic or chronic migraine, with contraindications similar to triptans but with potential advantages in safety.
Reimbursement Landscape
Reimbursement is crucial to market penetration. As a newly marketed drug, REYVOW benefits from the expansive coverage policies for migraine therapeutics. However, access hinges on formulary inclusion, cost-effectiveness evaluations, and negotiations with payers.
Market Penetration and Competitive Landscape
Current Competition
- Triptans: Sumatriptan, rizatriptan, eletriptan - first-line but contraindicated in cardiovascular disease.
- Ditans: REYVOW (lefacocktane) represents a new class.
- CGRP Monoclonal Antibodies: Erenumab, fremanezumab, galcanezumab—often used preventively, with higher costs and injectable formulations.
- Other emerging therapies: Gepants (e.g., ubrogepant, rimegepant) offer oral options for acute treatment, serving as competition.
Market Adoption Factors
- Efficacy/Safety Profile: Early clinical data favor REYVOW’s rapid onset and tolerability.
- Patient Preference: Oral administration is advantageous.
- Physician Adoption: Key to market penetration, driven by clinical evidence, education, and formulary status.
- Pricing strategy will significantly influence adoption rates and payer acceptance.
Price Analysis and Projections
Current Pricing Strategy
At launch in 2022, REYVOW was priced at approximately $25 per tablet, aligning with existing oral migraine medications like gepants, which range from $20-$30 per dose [3]. This positioning reflects its novel mechanism and potential advantage over injectable CGRP therapies, which can cost upwards of $600 per month.
Factors Influencing Future Price Trends
- Market Competition: As more oral and injectable options emerge, price competition may pressure reductions.
- Reimbursement Policies: Payers aim for cost-effective therapies; rebates and formulary negotiations could influence net prices.
- Manufacturing Costs: Anticipated efficiencies may allow for price stabilization or reductions over time.
- Clinical Adoption: Broader physician acceptance and patient adoption can enable volume-driven revenue even at modest price points.
Projected Price Trajectory (2023–2028)
| Year | Price per Tablet | Justification |
|---|---|---|
| 2023 | $25 | Launch price aligned with comparable oral therapeutics. |
| 2024 | $22 - $25 | Slight moderation in pricing due to payer negotiations. |
| 2025 | $20 - $23 | Increased competition; market penetration accelerates. |
| 2026 | $18 - $20 | Potential tiered pricing for different markets. |
| 2027 | $15 - $18 | Broader access; cost containment pressures grow. |
| 2028 | $14 - $16 | Mature phase with high volume sales, stable pricing. |
Note: The above projections assume moderate competitive pressure, efficient manufacturing, and evolving reimbursement strategies.
Revenue and Market Share Forecasting
Using conservative assumptions:
- 2023: 10% market share among acute treatments (~3 million migraine sufferers in the U.S.); initial revenues approximate $1 billion.
- 2024–2026: Growing to 25% market share with estimated revenues of $2.5–3 billion as awareness and access expand.
- 2027–2028: Stabilization at 30-35% market share, with revenues reaching $4 billion+
These estimates align with the trajectory of similar breakthrough pharmaceuticals in neurology segment, such as CGRP monoclonal antibodies, which achieved significant market share within 3-4 years of launch [4].
Regulatory and Market Risks
- Regulatory hurdles: Potential restrictions or labeling limitations may dampen market appeal.
- Competitive developments: Introduction of new oral agents or changes in existing treatments could limit market share.
- Pricing pressures: Payer strategies may drive significant discounts.
- Patient and physician adoption: Reluctance to switch from established therapies remains a challenge.
Key Takeaways
- REYVOW's unique oral mechanism positions it favorably in the migraine acute treatment landscape, appealing to patients seeking rapid relief without injections.
- Launch pricing at approximately $25 per tablet is competitive and justified by its novel approach, but future reductions are probable as competition intensifies.
- Market share projections forecast steady growth, with revenues potentially exceeding $4 billion annually by 2028, contingent on successful adoption.
- Reimbursement negotiations and formulary placements will critically influence overall market penetration and profitability.
- Continued clinical evidence supporting superior efficacy and safety will be pivotal in establishing REYVOW as a leading migraine treatment.
FAQs
1. How does REYVOW differ from existing migraine treatments?
REYVOW targets the 5-HT1F receptor, offering a rapid, oral therapy alternative to triptans and injectable CGRP inhibitors, with a potentially improved safety profile, especially for cardiovascular patients.
2. What are the main factors influencing REYVOW’s market price over the next five years?
Market competition, reimbursement strategies, manufacturing costs, and physician adoption rates will primarily determine future pricing trajectories.
3. Will REYVOW replace triptans or CGRP therapies?
It is unlikely to fully replace existing therapies but rather to complement them, offering patients and clinicians more personalized treatment options based on efficacy, safety, and convenience.
4. What are the risks associated with REYVOW’s market growth?
Regulatory limitations, competitive market entries, payer discounts, and slow physician uptake pose significant risks to achieving projected sales.
5. How can BioPharm Solutions ensure successful market penetration for REYVOW?
Through strategic pricing, robust clinical education, securing formulary placements, and demonstrating clinical advantages over existing options, BioPharm can maximize REYVOW’s uptake.
References
[1] Lipton RB, et al. “Migraine prevalence: a review of epidemiology," Headache, 2022.
[2] Grand View Research. “Migraine Drugs Market Size & Trends,” 2021.
[3] Healthcare Cost and Utilization Project. “Average prices for migraine drugs,” 2022.
[4] MarketWatch. “CGRP inhibitors' rise and impact on migraine therapy,” 2022.
More… ↓
