REXULTI Drug Patent Profile
✉ Email this page to a colleague
When do Rexulti patents expire, and when can generic versions of Rexulti launch?
Rexulti is a drug marketed by Otsuka and is included in one NDA. There are six patents protecting this drug and one Paragraph IV challenge.
This drug has seventy-seven patent family members in thirty-nine countries.
The generic ingredient in REXULTI is brexpiprazole. There are eight drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the brexpiprazole profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Rexulti
A generic version of REXULTI was approved as brexpiprazole by ALEMBIC on January 13th, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for REXULTI?
- What are the global sales for REXULTI?
- What is Average Wholesale Price for REXULTI?
Summary for REXULTI
| International Patents: | 77 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 11 |
| Patent Applications: | 1,143 |
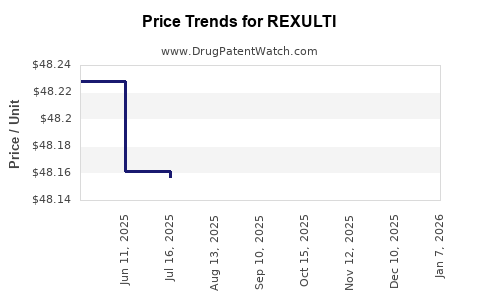
| Drug Prices: | Drug price information for REXULTI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for REXULTI |
| What excipients (inactive ingredients) are in REXULTI? | REXULTI excipients list |
| DailyMed Link: | REXULTI at DailyMed |
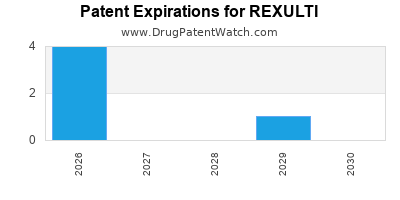
Recent Clinical Trials for REXULTI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dalhousie University | Phase 4 |
| University of Michigan | Phase 4 |
| Unity Health Toronto | Phase 4 |
Pharmacology for REXULTI
| Drug Class | Atypical Antipsychotic |
Paragraph IV (Patent) Challenges for REXULTI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| REXULTI | Tablets | brexpiprazole | 4 mg | 205422 | 16 | 2019-07-10 |
US Patents and Regulatory Information for REXULTI
REXULTI is protected by six US patents and five FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-004 | Jul 10, 2015 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-003 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-005 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-006 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-005 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-005 | Jul 10, 2015 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for REXULTI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Otsuka Pharmaceutical Netherlands B.V. | Rxulti | brexpiprazole | EMEA/H/C/003841Treatment of schizophrenia. | Authorised | no | no | no | 2018-07-26 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for REXULTI
When does loss-of-exclusivity occur for REXULTI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8319
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12321723
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014008603
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 51588
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14000909
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3889425
Estimated Expiration: ⤷ Get Started Free
Patent: 7397730
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 50480
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0200037
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 22460
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2930
Estimated Expiration: ⤷ Get Started Free
Patent: 1490783
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 47493
Estimated Expiration: ⤷ Get Started Free
India
Patent: 55DEN2014
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1513
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 2013054872
Estimated Expiration: ⤷ Get Started Free
Patent: 84161
Estimated Expiration: ⤷ Get Started Free
Patent: 17088610
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 53
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3370
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6092
Estimated Expiration: ⤷ Get Started Free
Patent: 14004135
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2639
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014500607
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 14013783
Estimated Expiration: ⤷ Get Started Free
Patent: 201608412S
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 67285
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1402333
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2072371
Estimated Expiration: ⤷ Get Started Free
Patent: 140075754
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 62479
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 34908
Estimated Expiration: ⤷ Get Started Free
Patent: 1318651
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4411
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering REXULTI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 2767285 | ⤷ Get Started Free | |
| Malaysia | 142746 | HETEROCYCLIC COMPOUND | ⤷ Get Started Free |
| Malaysia | 173370 | ⤷ Get Started Free | |
| European Patent Office | 2767285 | ⤷ Get Started Free | |
| Hungary | E011611 | ⤷ Get Started Free | |
| India | 855DEN2012 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for REXULTI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1869025 | SPC/GB18/038 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: BREXPIPRAZOLE OR A SALT THEREOF; REGISTERED: UK EU/1/18/1294/001(NI) 20180730; UK PLGB 506970019 20180730; UK PLGB 506970020 20180730; UK PLGB 506970021 20180730; UK PLGB 506970022 20180730; UK PLGB 506970023 20180730; UK EU/1/18/1294/007(NI) 20180730; UK EU/1/18/1294/008(NI) 20180730; UK EU/1/18/1294/009(NI) 20180730; UK EU/1/18/1294/010(NI) 20180730; UK EU/1/18/1294/011(NI) 20180730; UK PLGB 506970024 20180730; UK EU/1/18/1294/002(NI) 20180730; UK EU/1/18/1294/003(NI) 20180730; UK EU/1/18/1294/004(NI) 20180730; UK EU/1/18/1294/005(NI) 20180730; UK EU/1/18/1294/006(NI) 20180730 |
| 1869025 | 303 50013-2018 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: BREXPIPRAZOL VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/18/1294 20180730 |
| 1869025 | C20180022 00264 | Estonia | ⤷ Get Started Free | PRODUCT NAME: BREKSPIPRASOOL;REG NO/DATE: EU/1/18/1294 30.07.2018 |
| 1869025 | CA 2018 00028 | Denmark | ⤷ Get Started Free | PRODUCT NAME: BREXPIPRAZOL ELLER ET SALT DERAF; REG. NO/DATE: EU/1/18/1294/001-006 20180727 |
| 1869025 | C201830050 | Spain | ⤷ Get Started Free | PRODUCT NAME: BREXPIPRAZOL O UNA SAL DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/18/1294; DATE OF AUTHORISATION: 20180726; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1294; DATE OF FIRST AUTHORISATION IN EEA: 20180726 |
| 1869025 | 300946 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BREXPIPRAZOLE, DESGEWENST IN DE VORM VAN EEN ZOUT; REGISTRATION NO/DATE: EU/1/18/1294 20180727 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for REXULTI (Brexpiprazole)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
