RECORLEV Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Recorlev, and when can generic versions of Recorlev launch?
Recorlev is a drug marketed by Strongbridge and is included in one NDA. There are eight patents protecting this drug.
This drug has thirty-three patent family members in twenty-one countries.
The generic ingredient in RECORLEV is levoketoconazole. One supplier is listed for this compound. Additional details are available on the levoketoconazole profile page.
DrugPatentWatch® Generic Entry Outlook for Recorlev
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 30, 2028. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for RECORLEV?
- What are the global sales for RECORLEV?
- What is Average Wholesale Price for RECORLEV?
Summary for RECORLEV
| International Patents: | 33 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 47 |
| Patent Applications: | 1,512 |
| Drug Prices: | Drug price information for RECORLEV |
| What excipients (inactive ingredients) are in RECORLEV? | RECORLEV excipients list |
| DailyMed Link: | RECORLEV at DailyMed |
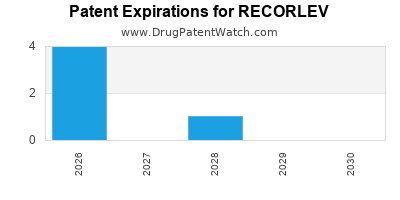

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for RECORLEV
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for RECORLEV
US Patents and Regulatory Information for RECORLEV
RECORLEV is protected by eight US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of RECORLEV is ⤷ Sign Up.
This potential generic entry date is based on FOR TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN ADULT PATIENTS WITH CUSHING'S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting RECORLEV
Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN PATIENTS WITH CUSHING’S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN PATIENTS WITH CUSHING’S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN PATIENTS WITH CUSHING’S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Methods of treating disease with levoketoconazole
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: DURING LEVOKETOCONAZOLE DOSAGE TITRATION FOR THE TREATMENT OF CUSHING'S SYNDROME IN PATIENTS WHO CONCOMITANTLY USE METFORMIN, MONITORING GLYCEMIA, KIDNEY FUNCTION AND VITAMIN B-12 AND ADJUSTING DOSAGE OF METFORMIN AS NEEDED
Methods of treating disease with levoketoconazole
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: DURING LEVOKETOCONAZOLE DOSAGE TITRATION FOR THE TREATMENT OF CUSHING'S SYNDROME IN PATIENTS WHO CONCOMITANTLY USE METFORMIN, MONITORING GLYCEMIA, KIDNEY FUNCTION AND VITAMIN B-12 AND ADJUSTING DOSAGE OF METFORMIN AS NEEDED
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN PATIENTS WITH CUSHING’S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: DURING LEVOKETOCONAZOLE DOSAGE TITRATION FOR THE TREATMENT OF CUSHING'S SYNDROME IN PATIENTS WHO CONCOMITANTLY USE AN OCT2 SUBSTRATE, MONITORING THE SUBJECT FOR A DOSE LIMITING EVENT AND ADJUSTING THE DOSAGE OF THE OCT2 SUBSTRATE AS NEEDED
Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN PATIENTS WITH CUSHING’S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
FDA Regulatory Exclusivity protecting RECORLEV
FOR TREATMENT OF ENDOGENOUS HYPERCORTISOLEMIA IN ADULT PATIENTS WITH CUSHING'S SYNDROME FOR WHOM SURGERY IS NOT AN OPTION OR HAS NOT BEEN CURATIVE
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strongbridge | RECORLEV | levoketoconazole | TABLET;ORAL | 214133-001 | Dec 30, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Strongbridge | RECORLEV | levoketoconazole | TABLET;ORAL | 214133-001 | Dec 30, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Strongbridge | RECORLEV | levoketoconazole | TABLET;ORAL | 214133-001 | Dec 30, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Strongbridge | RECORLEV | levoketoconazole | TABLET;ORAL | 214133-001 | Dec 30, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Strongbridge | RECORLEV | levoketoconazole | TABLET;ORAL | 214133-001 | Dec 30, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for RECORLEV
When does loss-of-exclusivity occur for RECORLEV?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06204334
Patent: Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 28005
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 94433
Patent: PROCEDES ET COMPOSITIONS POUR LE TRAITEMENT DU DIABETE, DU SYNDROME METABOLIQUE ET D'AUTRES CONDITIONS (METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS)
Estimated Expiration: ⤷ Sign Up
China
Patent: 1141964
Patent: Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 12519
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 53266
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 53266
Patent: 2S,4R KETOCONAZOLE POUR LE TRAITEMENT DU DIABETE, DU SYNDROME METABOLIQUE ET D'AUTRES CONDITIONS (2S,4R KETOCONAZOLE FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS)
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 18449
Patent: METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 4459
Patent: תכשירי רוקחות המכילים אנאנטיאומר של s2, r4 קטוקונאזול לטיפול במחלות הנובעות מהגברת קורטיזול (Use of a 2s, 4r ketoconazole enantiomer for the preparation of medicaments for the treatment of elevated cortisol-mediated diseases)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 58095
Estimated Expiration: ⤷ Sign Up
Patent: 08526830
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 07008331
Patent: METODOS Y COMPOSICIONES PARA EL TRATAMIENTO DE DIABETES, SINDROME METABOLICO Y OTRAS CONDICIONES. (METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS.)
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0481
Patent: Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 9007
Estimated Expiration: ⤷ Sign Up
Patent: 074117
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 53266
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 53266
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 53266
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0706020
Patent: Methods and compositions for treating diabetes, metabolic syndrome and other conditions
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1387910
Estimated Expiration: ⤷ Sign Up
Patent: 070100781
Patent: METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS
Estimated Expiration: ⤷ Sign Up
Patent: 140030327
Patent: METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITION
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 77526
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RECORLEV around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20070100781 | METHODS AND COMPOSITIONS FOR TREATING DIABETES, METABOLIC SYNDROME AND OTHER CONDITIONS | ⤷ Sign Up |
| Australia | 2020232246 | Methods of treating disease with levoketoconazole | ⤷ Sign Up |
| Spain | 2377526 | ⤷ Sign Up | |
| Japan | 5358095 | ⤷ Sign Up | |
| Canada | 3132319 | PROCEDES DE TRAITEMENT D'UNE MALADIE AVEC DU LEVOKETOCONAZOLE (METHODS OF TREATING DISEASE WITH LEVOKETOCONAZOLE) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


