RAYOS Drug Patent Profile
✉ Email this page to a colleague
When do Rayos patents expire, and what generic alternatives are available?
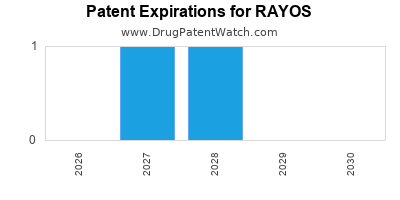
Rayos is a drug marketed by Horizon and is included in one NDA. There are six patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-three patent family members in twenty-nine countries.
The generic ingredient in RAYOS is prednisone. There are sixteen drug master file entries for this compound. Forty-eight suppliers are listed for this compound. Additional details are available on the prednisone profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Rayos
A generic version of RAYOS was approved as prednisone by WATSON LABS on December 31st, 1969.
Summary for RAYOS
| International Patents: | 53 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 89 |
| Patent Applications: | 4,584 |
| Formulation / Manufacturing: | see details |
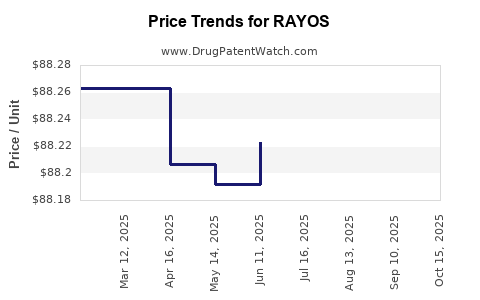
| Drug Prices: | Drug price information for RAYOS |
| What excipients (inactive ingredients) are in RAYOS? | RAYOS excipients list |
| DailyMed Link: | RAYOS at DailyMed |
Pharmacology for RAYOS
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for RAYOS
Paragraph IV (Patent) Challenges for RAYOS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RAYOS | Delayed-release Tablets | prednisone | 5 mg | 202020 | 1 | 2012-11-26 |
US Patents and Regulatory Information for RAYOS
RAYOS is protected by seven US patents.
Patents protecting RAYOS
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF RHEUMATOLOGIC, ALLERGIC, PULMONARY,GASTROINTESTINAL,DERMATOLOGIC DISEASES OR CONDITIONS BY THE USE OF A DELAYED RELEASE 5MG PREDNISONE TABLET
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DISEASES OR CONDITIONS BY THE USE OF A DELAYED RELEASE 1, 2, OR 5 MG PREDNISONE TABLET
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DISEASES OR CONDITIONS BY THE USE OF A DELAYED-RELEASE 1,2, OR 5MG PREDNISONE TABLET
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DISEASES OR CONDITIONS BY THE USE OF A DELAYED-RELEASE 1,2, OR 5MG PREDNISONE TABLET
Delayed release tablet with defined core geometry
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DISEASES OR CONDITIONS BY THE USE OF A DELAYED-RELEASE 1,2, OR 5MG PREDNISONE TABLET
Delayed-release glucocorticoid treatment of rheumatoid disease
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF DISEASES OR CONDITIONS BY THE USE OF A DELAYED-RELEASE 1,2, OR 5MG PREDNISONE TABLET
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-002 | Jul 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-003 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-002 | Jul 26, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-003 | Jul 26, 2012 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RAYOS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-002 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-003 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-002 | Jul 26, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for RAYOS
See the table below for patents covering RAYOS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2006524229 | ⤷ Sign Up | |
| China | 1777412 | Delayed release tablet with defined core geometry | ⤷ Sign Up |
| Denmark | 1631251 | ⤷ Sign Up | |
| Malaysia | 149863 | DELAYED-RELEASE GLUCOCORTICOID TREATMENT OF RHEUMATOID DISEASE | ⤷ Sign Up |
| Canada | 2361503 | ⤷ Sign Up | |
| Slovenia | 2049123 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


