RAYALDEE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Rayaldee, and what generic alternatives are available?
Rayaldee is a drug marketed by Eirgen and is included in one NDA. There are sixteen patents protecting this drug.
This drug has one hundred and eighty-five patent family members in thirty-eight countries.
The generic ingredient in RAYALDEE is calcifediol. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the calcifediol profile page.
DrugPatentWatch® Generic Entry Outlook for Rayaldee
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 14, 2034. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for RAYALDEE?
- What are the global sales for RAYALDEE?
- What is Average Wholesale Price for RAYALDEE?
Summary for RAYALDEE
| International Patents: | 185 |
| US Patents: | 16 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 75 |
| Clinical Trials: | 1 |
| Patent Applications: | 3,471 |
| Drug Prices: | Drug price information for RAYALDEE |
| What excipients (inactive ingredients) are in RAYALDEE? | RAYALDEE excipients list |
| DailyMed Link: | RAYALDEE at DailyMed |
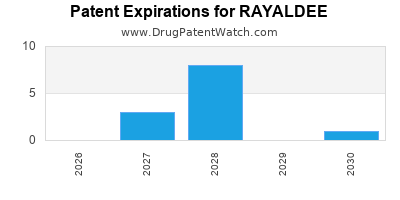
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for RAYALDEE
Generic Entry Date for RAYALDEE*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE, EXTENDED RELEASE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for RAYALDEE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| OPKO Health, Inc. | Phase 2 |
Pharmacology for RAYALDEE
| Drug Class | Vitamin D3 Analog |
US Patents and Regulatory Information for RAYALDEE
RAYALDEE is protected by twenty-two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of RAYALDEE is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,861,644.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 8,778,373 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 10,300,078 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 9,925,147 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 10,213,442 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 8,426,391 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 10,357,502 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | 9,861,644 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RAYALDEE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | 6,582,727 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for RAYALDEE
When does loss-of-exclusivity occur for RAYALDEE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5576
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 14228069
Estimated Expiration: ⤷ Get Started Free
Patent: 19200268
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015023658
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 05409
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 15002659
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5246464
Estimated Expiration: ⤷ Get Started Free
Patent: 1346071
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 190178
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0201284
Estimated Expiration: ⤷ Get Started Free
Patent: 0201869
Estimated Expiration: ⤷ Get Started Free
Patent: 0211265
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 23167
Estimated Expiration: ⤷ Get Started Free
Patent: 23568
Estimated Expiration: ⤷ Get Started Free
Patent: 24393
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 23024864
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8867
Estimated Expiration: ⤷ Get Started Free
Patent: 1591809
Estimated Expiration: ⤷ Get Started Free
Patent: 1991774
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
Patent: 88638
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 20128
Estimated Expiration: ⤷ Get Started Free
Patent: 20362
Estimated Expiration: ⤷ Get Started Free
Patent: 56895
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 51923
Estimated Expiration: ⤷ Get Started Free
Patent: 52014
Estimated Expiration: ⤷ Get Started Free
Patent: 55591
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1456
Estimated Expiration: ⤷ Get Started Free
Patent: 4841
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 92051
Estimated Expiration: ⤷ Get Started Free
Patent: 33268
Estimated Expiration: ⤷ Get Started Free
Patent: 82832
Estimated Expiration: ⤷ Get Started Free
Patent: 16517429
Estimated Expiration: ⤷ Get Started Free
Patent: 18012737
Estimated Expiration: ⤷ Get Started Free
Patent: 19135264
Estimated Expiration: ⤷ Get Started Free
Patent: 21155460
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4092
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 15012625
Estimated Expiration: ⤷ Get Started Free
Patent: 20011736
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1924
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 21007
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 151761
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 015502162
Estimated Expiration: ⤷ Get Started Free
Patent: 021551127
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 5361134
Patent: صيغة مستقرة ذات إطلاق متحكم فيه لمركب فيتامين د وطريقة إعطائها (Stabilized controlled release formulation of compound vitamin d and method of administering same)
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 846
Patent: STABILIZOVANA FORMULACIJA VITAMINA D SA MODIFIKOVANIM OSLOBAĐANJEM I POSTUPAK ZA DAVANJE ISTE (STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTRING SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 132
Patent: STABILIZOVANA FORMULACIJA VITAMINA D SA MODIFIKOVANIM OSLOBAĐANJEM I POSTUPAK ZA NJENU PRIMENU (STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTERING SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 176
Patent: STABILIZOVANA FORMULACIJA VITAMINA D SA MODIFIKOVANIM OSLOBAĐANJEM I POSTUPAK ZA NJENU PRIMENU (STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTERING SAME)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201703517V
Patent: STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTERING SAME
Estimated Expiration: ⤷ Get Started Free
Patent: 201507323P
Patent: STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTRING SAME
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 68172
Estimated Expiration: ⤷ Get Started Free
Patent: 32773
Estimated Expiration: ⤷ Get Started Free
Patent: 50016
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1847947
Estimated Expiration: ⤷ Get Started Free
Patent: 2203003
Estimated Expiration: ⤷ Get Started Free
Patent: 140113374
Estimated Expiration: ⤷ Get Started Free
Patent: 140140004
Estimated Expiration: ⤷ Get Started Free
Patent: 190095216
Estimated Expiration: ⤷ Get Started Free
Patent: 210078463
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 09477
Estimated Expiration: ⤷ Get Started Free
Patent: 34900
Estimated Expiration: ⤷ Get Started Free
Patent: 82567
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 59753
Estimated Expiration: ⤷ Get Started Free
Patent: 1707689
Patent: Stabilized modified release vitamin D formulation and method of administering same
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 3386
Patent: СТАБІЛІЗОВАНИЙ СКЛАД ВІТАМІНУ D ІЗ МОДИФІКОВАНИМ ВИВІЛЬНЕННЯМ І СПОСІБ ЙОГО ВВЕДЕННЯ (STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTRING SAME)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RAYALDEE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 2020043 | ⤷ Get Started Free | |
| Japan | 2014065751 | ⤷ Get Started Free | |
| South Korea | 20140113374 | STABILIZED MODIFIED RELEASE VITAMIN D FORMULATION AND METHOD OF ADMINISTERING SAME | ⤷ Get Started Free |
| Peru | 20151761 | FORMULACION DE VITAMINA D DE LIBERACION MODIFICADA Y ESTABILIZADA Y METODO DE ADMINISTRACION DE LA MISMA | ⤷ Get Started Free |
| Malaysia | 200794 | ⤷ Get Started Free | |
| Cyprus | 1115567 | ⤷ Get Started Free | |
| Spain | 2496915 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RAYALDEE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2481400 | 132021000000071 | Italy | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOLO(RAYALDEE); AUTHORISATION NUMBER(S) AND DATE(S): 047870011, 20201201;PL 50784/0005, 20200721 |
| 2481400 | C202130022 | Spain | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOL; NATIONAL AUTHORISATION NUMBER: 85519-DE/H/5590/001/DC; DATE OF AUTHORISATION: 20201230; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): DE/H/5590/001/DC; DATE OF FIRST AUTHORISATION IN EEA: 20200721 |
| 2968172 | CA 2021 00005 | Denmark | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOL; NAT. REG. NO/DATE: 62564 20200910; FIRST REG. NO/DATE: DE 2202115.00.00 20200818 |
| 2481400 | CR 2020 00059 | Denmark | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOL; NAT. REG. NO/DATE: 62564 20200910; FIRST REG. NO/DATE: DE 2202115.00.00 20200818 |
| 2481400 | 301085 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOL IN IEDERE VORM ZOALS BESCHERMD DOOR HET BASISOCTROOI; NATIONAL REGISTRATION NO/DATE: 124799 20200922; FIRST REGISTRATION: DE 2202115.00.00 20200819 |
| 2968172 | CR 2021 00005 | Denmark | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOL; NAT. REG. NO/DATE: 62564 20200910; FIRST REG. NO/DATE: DE 2202115.00.00 20200818 |
| 2968172 | 132021000000074 | Italy | ⤷ Get Started Free | PRODUCT NAME: CALCIFEDIOLO(RAYALDEE); AUTHORISATION NUMBER(S) AND DATE(S): 047870011, 20201201;PL 50784/0005, 20200721 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for RAYALDEE (Dryad Data: SD-1016)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
