PYLARIFY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Pylarify, and what generic alternatives are available?
Pylarify is a drug marketed by Progenics Pharms Inc and is included in one NDA. There are five patents protecting this drug.
This drug has ninety-six patent family members in twenty-four countries.
The generic ingredient in PYLARIFY is piflufolastat f-18. One supplier is listed for this compound. Additional details are available on the piflufolastat f-18 profile page.
DrugPatentWatch® Generic Entry Outlook for Pylarify
Pylarify will be eligible for patent challenges on May 26, 2025. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 21, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for PYLARIFY?
- What are the global sales for PYLARIFY?
- What is Average Wholesale Price for PYLARIFY?
Summary for PYLARIFY
| International Patents: | 96 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 3 |
| Clinical Trials: | 5 |
| Patent Applications: | 22 |
| Drug Prices: | Drug price information for PYLARIFY |
| What excipients (inactive ingredients) are in PYLARIFY? | PYLARIFY excipients list |
| DailyMed Link: | PYLARIFY at DailyMed |
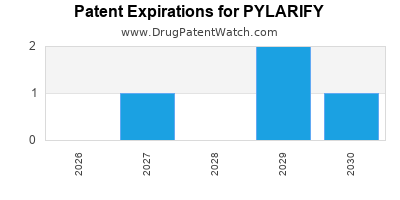

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PYLARIFY
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PYLARIFY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| OHSU Knight Cancer Institute | Phase 4 |
| Progenics Pharmaceuticals, Inc. | Phase 4 |
| Oregon Health and Science University | Phase 4 |
Pharmacology for PYLARIFY
| Drug Class | Radioactive Diagnostic Agent |
| Mechanism of Action | Positron Emitting Activity |
US Patents and Regulatory Information for PYLARIFY
PYLARIFY is protected by five US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PYLARIFY is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PYLARIFY
Synthesis of the radiolabeled prostate-specific membrane antigen (PSMA) inhibitor [.sup.18F]DCFPyL
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF POSITRON EMISSION TOMOGRAPHY (PET) IN MEN WITH PROSTATE CANCER
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF POSITRON EMISSION TOMOGRAPHY (PET) IN MEN WITH PROSTATE CANCER
Heterodimers of glutamic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
PSMA-binding agents and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF POSITRON EMISSION TOMOGRAPHY (PET) IN MEN WITH PROSTATE CANCER
PSMA-binding agents and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF POSITRON EMISSION TOMOGRAPHY (PET) IN MEN WITH PROSTATE CANCER
FDA Regulatory Exclusivity protecting PYLARIFY
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progenics Pharms Inc | PYLARIFY | piflufolastat f-18 | SOLUTION;INTRAVENOUS | 214793-001 | May 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Progenics Pharms Inc | PYLARIFY | piflufolastat f-18 | SOLUTION;INTRAVENOUS | 214793-001 | May 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Progenics Pharms Inc | PYLARIFY | piflufolastat f-18 | SOLUTION;INTRAVENOUS | 214793-001 | May 26, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PYLARIFY
When does loss-of-exclusivity occur for PYLARIFY?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09276423
Patent: PSMA-binding agents and uses thereof
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 32632
Patent: AGENTS DE LIAISON AU PSMA ET SES UTILISATIONS (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 87744
Patent: AGENTS DE LIAISON AU PSMA ET SES UTILISATIONS (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
China
Patent: 2171187
Patent: PSMA-binding agents and uses thereof
Estimated Expiration: ⤷ Sign Up
Patent: 7382846
Patent: PSMA‑结合剂及其用途 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 3563262
Patent: PSMA-结合剂及其用途 (PSMA-binding agents and uses thereof)
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0171133
Estimated Expiration: ⤷ Sign Up
Patent: 0220742
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 19367
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 18366
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 18366
Patent: AGENTS DE LIAISON AU PSMA ET SES UTILISATIONS (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Patent: AGENTS SE LIANT AU PSMA ET LEURS UTILISATIONS (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 89074
Patent: AGENTS SE LIANT AU PSMA ET LEURS UTILISATIONS (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 0230033
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 47197
Patent: PSMA-結合劑及其用途 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 34027
Estimated Expiration: ⤷ Sign Up
Patent: 59436
Estimated Expiration: ⤷ Sign Up
Patent: 300039
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 88441
Estimated Expiration: ⤷ Sign Up
Patent: 06765
Estimated Expiration: ⤷ Sign Up
Patent: 30724
Estimated Expiration: ⤷ Sign Up
Patent: 24046
Estimated Expiration: ⤷ Sign Up
Patent: 19497
Estimated Expiration: ⤷ Sign Up
Patent: 77113
Estimated Expiration: ⤷ Sign Up
Patent: 11529919
Estimated Expiration: ⤷ Sign Up
Patent: 15007058
Patent: PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 16053025
Patent: PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 17048204
Patent: PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 19055973
Patent: PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Patent: 21095407
Patent: PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF)
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 18366
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 1250
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 24006
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 18366
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 18366
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 94096
Patent: АГЕНТЫ, СВЯЗЫВАЮЩИЕСЯ С PSMA, И ИХ ПРИМЕНЕНИЕ (PSMA-BINDING AGENTS AND USING THEM)
Estimated Expiration: ⤷ Sign Up
Patent: 11107752
Patent: АГЕНТЫ, СВЯЗЫВАЮЩИЕСЯ С PSMA, И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 18366
Estimated Expiration: ⤷ Sign Up
Patent: 22615
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1664855
Estimated Expiration: ⤷ Sign Up
Patent: 110038725
Patent: PSMA-BINDING AGENTS AND USES THEREOF
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 34894
Estimated Expiration: ⤷ Sign Up
Patent: 14593
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PYLARIFY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2021095407 | PSMA結合剤及びその使用 (PSMA-BINDING AGENTS AND USES THEREOF) | ⤷ Sign Up |
| South Korea | 102558962 | ⤷ Sign Up | |
| Spain | 2684322 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PYLARIFY
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2318366 | 301250 | Netherlands | ⤷ Sign Up | PRODUCT NAME: PIFLUFOLASTAT (18F) OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/23/1746 20230725 |
| 2318366 | LUC00323 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: PIFLUFOLASTAT (18F) OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/23/1746 20230725 |
| 2318366 | 2023C/540 | Belgium | ⤷ Sign Up | PRODUCT NAME: PIFLUFOLASTAT (18F) OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/23/1746 20230725 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


