PROMACTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Promacta, and what generic alternatives are available?
Promacta is a drug marketed by Novartis and is included in two NDAs. There are six patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and eight patent family members in forty countries.
The generic ingredient in PROMACTA is eltrombopag olamine. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the eltrombopag olamine profile page.
DrugPatentWatch® Generic Entry Outlook for Promacta
Promacta was eligible for patent challenges on November 20, 2012.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 1, 2028. This may change due to patent challenges or generic licensing.
There are two Paragraph IV patent challenges for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for PROMACTA
| International Patents: | 108 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 72 |
| Clinical Trials: | 31 |
| Patent Applications: | 319 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PROMACTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROMACTA |
| What excipients (inactive ingredients) are in PROMACTA? | PROMACTA excipients list |
| DailyMed Link: | PROMACTA at DailyMed |
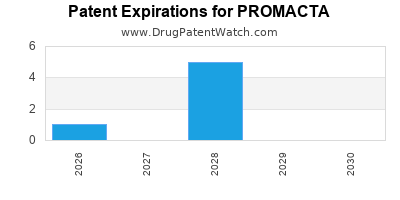

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PROMACTA
Generic Entry Date for PROMACTA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PROMACTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of California, San Francisco | Phase 1 |
| Food and Drug Administration (FDA) | Phase 1 |
| University of California, Davis | Phase 1 |
Pharmacology for PROMACTA
Anatomical Therapeutic Chemical (ATC) Classes for PROMACTA
Paragraph IV (Patent) Challenges for PROMACTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROMACTA | Tablets | eltrombopag olamine | 12.5 mg and 25 mg | 022291 | 1 | 2014-02-04 |
| PROMACTA | Tablets | eltrombopag olamine | 50 mg and 75 mg | 022291 | 1 | 2014-01-07 |
US Patents and Regulatory Information for PROMACTA
PROMACTA is protected by six US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PROMACTA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PROMACTA
3'-[(2z)-[1-(3,4-Dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4h-pyrazol-4-y- lidene]hy-drazino]-2'-hydroxy-[1,1'-piphenyl]-acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3'-[(2Z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-y- lidene]hydrazino]-2'-hydroxy[1,1'-biphenyl]-3-carboxylic acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3'-[(2Z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-y- lidene] hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3'-[(2Z)-[1-(3,4-dimethylphenyl)-1 ,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1- '-biphenyl]-3-carboxylic acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3'-[(2Z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-y- lidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3'-[(2Z)-[1-(3,4-dimethylphenyl)-1,5-dihydro-3-methyl-5-oxo-4H-pyrazol-4-y- lidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid bis-(monoethanolamine)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting PROMACTA
INDICATED IN COMBINATION WITH STANDARD IMMUNOSUPPRESSIVE THERAPY FOR THE FIRST-LINE TREATMENT OF ADULT AND PEDIATRIC PATIENTS 2 YEARS AND OLDER WITH SEVERE APLASTIC ANEMIA
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | PROMACTA KIT | eltrombopag olamine | FOR SUSPENSION;ORAL | 207027-002 | Sep 27, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-003 | Sep 8, 2009 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | PROMACTA KIT | eltrombopag olamine | FOR SUSPENSION;ORAL | 207027-001 | Aug 24, 2015 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-005 | Nov 16, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PROMACTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-001 | Nov 20, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-004 | Oct 20, 2011 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-002 | Nov 20, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-004 | Oct 20, 2011 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PROMACTA
When does loss-of-exclusivity occur for PROMACTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9656
Estimated Expiration: ⤷ Try a Trial
Patent: 7711
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 07352608
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0721651
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 85831
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 07002242
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1686930
Estimated Expiration: ⤷ Try a Trial
Patent: 2688207
Estimated Expiration: ⤷ Try a Trial
Patent: 2697745
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 60058
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 143
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0160206
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 17284
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 52237
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 009000253
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 077628
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 0883
Estimated Expiration: ⤷ Try a Trial
Patent: 4294
Estimated Expiration: ⤷ Try a Trial
Patent: 0971018
Estimated Expiration: ⤷ Try a Trial
Patent: 1400387
Estimated Expiration: ⤷ Try a Trial
Patent: 1991590
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 52237
Estimated Expiration: ⤷ Try a Trial
Patent: 90730
Estimated Expiration: ⤷ Try a Trial
Patent: 18732
Estimated Expiration: ⤷ Try a Trial
Patent: 18733
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 36968
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 27209
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 1891
Estimated Expiration: ⤷ Try a Trial
Patent: 8840
Estimated Expiration: ⤷ Try a Trial
Patent: 4602
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 19866
Estimated Expiration: ⤷ Try a Trial
Patent: 35078
Estimated Expiration: ⤷ Try a Trial
Patent: 44713
Estimated Expiration: ⤷ Try a Trial
Patent: 60289
Estimated Expiration: ⤷ Try a Trial
Patent: 10526140
Estimated Expiration: ⤷ Try a Trial
Patent: 14005302
Estimated Expiration: ⤷ Try a Trial
Patent: 15129195
Estimated Expiration: ⤷ Try a Trial
Patent: 17137343
Estimated Expiration: ⤷ Try a Trial
Patent: 19123747
Estimated Expiration: ⤷ Try a Trial
Patent: 21100968
Estimated Expiration: ⤷ Try a Trial
Patent: 23011888
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 43
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 8072
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 09011881
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 236
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 0888
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 080773
Estimated Expiration: ⤷ Try a Trial
Patent: 121407
Estimated Expiration: ⤷ Try a Trial
Patent: 151953
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 52237
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 52237
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 52237
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0907710
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1475971
Estimated Expiration: ⤷ Try a Trial
Patent: 1537200
Estimated Expiration: ⤷ Try a Trial
Patent: 1632851
Estimated Expiration: ⤷ Try a Trial
Patent: 100020456
Estimated Expiration: ⤷ Try a Trial
Patent: 140049086
Estimated Expiration: ⤷ Try a Trial
Patent: 150008513
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 65179
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 39267
Estimated Expiration: ⤷ Try a Trial
Patent: 38674
Estimated Expiration: ⤷ Try a Trial
Patent: 0843742
Estimated Expiration: ⤷ Try a Trial
Patent: 1410240
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 261
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PROMACTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Israel | 193617 | חיקויים של תרומבופויאטין, תכשירי רוקחות המכילים אותם ושימושם בהכנת תרופות לטיפול בתרומבוציטופניה (Thrombopoietin mimetics, pharmaceutical compositions comprising them and use thereof in the preparation of medicaments for treating thrombocytopenia) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2008136843 | ⤷ Try a Trial | |
| Lithuania | PA2010007 | ⤷ Try a Trial | |
| Poland | 2152237 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROMACTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1534390 | C20100006 | Estonia | ⤷ Try a Trial | PRODUCT NAME: REVOLADE-ELTROMBOPAG; AUTHORISATIN NO.: EMA/CHMP/697489/2018; AUTHORISATION DATE: 20181019 |
| 1294378 | SPC/GB10/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ELTROMBOPAG, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE (INCLUDING A HYDRATE); REGISTERED: UK EU1/10/612/001 20100315; UK EU1/10/612/002 20100315; UK EU1/10/612/003 20100315; UK EU1/10/612/004 20100315; UK EU1/10/612/005 20100315; UK EU1/10/612/006 20100315 |
| 1534390 | C 2010 013 | Romania | ⤷ Try a Trial | PRODUCT NAME: ELTROMBOPAG; NATIONAL AUTHORISATION NUMBER: RO EU/1/10/612/001, RO EU/1/10/612/002, RO EU/1/10/612/003, RO EU/1/10/612/004, RO EU/1/10/612/005, RO EU/1/10/612/006; DATE OF NATIONAL AUTHORISATION: 20100311; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/10/612/001, EMEA EU/1/10/612/002, EMEA EU/1/10/612/003, EMEA EU/1/10/612/004, EMEA EU/1/10/612/005, EMEA EU/1/10/612/006; DATE OF FIRST AUTHORISATION IN EEA: 20100311 |
| 1534390 | C20100006 00032 | Estonia | ⤷ Try a Trial | PRODUCT NAME: REVOLADE-ELTROMBOPAG; REG NO/DATE: C(2010)1662 11.03.2010 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


