PROLENSA Drug Patent Profile
✉ Email this page to a colleague
When do Prolensa patents expire, and when can generic versions of Prolensa launch?
Prolensa is a drug marketed by Bausch And Lomb and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has twenty-four patent family members in thirteen countries.
The generic ingredient in PROLENSA is bromfenac sodium. Ten suppliers are listed for this compound. Additional details are available on the bromfenac sodium profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Prolensa
A generic version of PROLENSA was approved as bromfenac sodium by SENTISS on January 22nd, 2014.
Summary for PROLENSA
| International Patents: | 24 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 88 |
| Clinical Trials: | 5 |
| Patent Applications: | 5,335 |
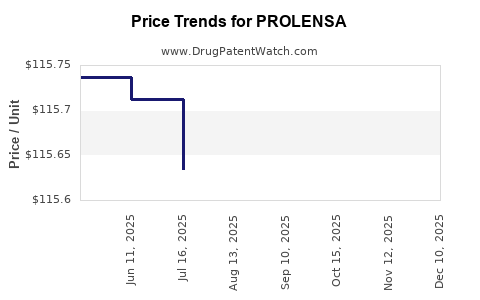
| Drug Prices: | Drug price information for PROLENSA |
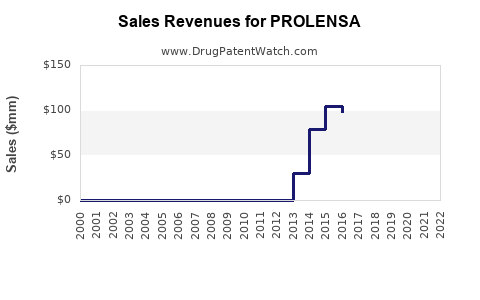
| Drug Sales Revenues: | Drug sales revenues for PROLENSA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROLENSA |
| What excipients (inactive ingredients) are in PROLENSA? | PROLENSA excipients list |
| DailyMed Link: | PROLENSA at DailyMed |
Recent Clinical Trials for PROLENSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wyse Eyecare | Phase 4 |
| Ocular Therapeutix, Inc. | Phase 4 |
| Sight Medical Doctors PLLC | Phase 4 |
Pharmacology for PROLENSA
| Drug Class | Nonsteroidal Anti-inflammatory Drug |
| Mechanism of Action | Cyclooxygenase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for PROLENSA
Paragraph IV (Patent) Challenges for PROLENSA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROLENSA | Ophthalmic Solution | bromfenac sodium | 0.07% | 203168 | 1 | 2013-07-26 |
US Patents and Regulatory Information for PROLENSA
PROLENSA is protected by ten US patents.
Patents protecting PROLENSA
Bromfenac bioavailability
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING OCULAR INFLAMMATION
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATMENT TO ALLEVIATE INFLAMMATION OF THE EYE
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Bromfenac bioavailability
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POSTOPERATIVE INFLAMMATION AND REDUCTION OF OCULAR PAIN IN PATIENTS WHO HAVE UNDERGONE CATARACT SURGERY
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POSTOPERATIVE INFLAMMATION AND REDUCTION OF OCULAR PAIN IN PATIENTS WHO HAVE UNDERGONE CATARACT SURGERY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PROLENSA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb | PROLENSA | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 203168-001 | Apr 5, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PROLENSA
See the table below for patents covering PROLENSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2765988 | COMPOSITION CONTENANT BROMOFENAC À L'ADMINISTRATION À VOIE OCULAIRE AVEC UNE BIODISPONIBILITÉ AUGMENTÉ (OCULAR COMPOSITION CONTAINING BROMFENAC WITH INCREASED BIOAVAILABILITY) | ⤷ Try a Trial |
| Mexico | 348418 | COMPOSICION OCULAR QUE CONTIENE BROMFENACO CON BIODISPONIBILIDAD INCREMENTADA. (OCULAR COMPOSITION CONTAINING BROMFENAC WITH INCREASED BIOAVAILABILITY.) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2015170177 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROLENSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1586316 | 1190018-0 | Sweden | ⤷ Try a Trial | RAETTELSE AV SKYDDSTID: 2024-01-17 TILL OCH MED DEN 2026-05-22 |
| 1586316 | 122011100019 | Germany | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC (2-AMINO-3-(4-BROMOBENZOYL)PHENYLESSIGSAEURE); REGISTRATION NO/DATE: EU/1/11/692/001 20110518 |
| 1586316 | 11C0031 | France | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC, SES SELS OU HYDRATES PHARMACOLOGIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/11/692/001 20110518 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


