PROCYSBI Drug Patent Profile
✉ Email this page to a colleague
When do Procysbi patents expire, and when can generic versions of Procysbi launch?
Procysbi is a drug marketed by Horizon and is included in two NDAs. There are twelve patents protecting this drug and two Paragraph IV challenges.
This drug has sixty-two patent family members in thirty-four countries.
The generic ingredient in PROCYSBI is cysteamine bitartrate. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the cysteamine bitartrate profile page.
DrugPatentWatch® Generic Entry Outlook for Procysbi
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 17, 2034. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for PROCYSBI
| International Patents: | 62 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 13 |
| Clinical Trials: | 1 |
| Patent Applications: | 15 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PROCYSBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROCYSBI |
| What excipients (inactive ingredients) are in PROCYSBI? | PROCYSBI excipients list |
| DailyMed Link: | PROCYSBI at DailyMed |
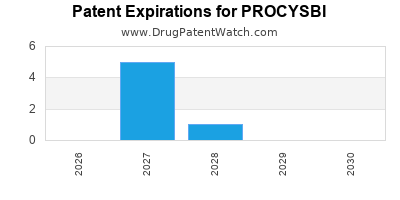

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PROCYSBI
Generic Entry Dates for PROCYSBI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE, DELAYED RELEASE;ORAL |
Generic Entry Dates for PROCYSBI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
GRANULE, DELAYED RELEASE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PROCYSBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Raptor Pharmaceuticals Inc. | Phase 3 |
| Horizon Pharma USA, Inc. | Phase 3 |
Pharmacology for PROCYSBI
| Drug Class | Cystine Depleting Agent |
| Mechanism of Action | Cystine Disulfide Reduction |
Paragraph IV (Patent) Challenges for PROCYSBI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROCYSBI | Delayed-release Granules | cysteamine bitartrate | 75 mg/Packet and 300 mg/Packet | 213491 | 1 | 2021-12-16 |
| PROCYSBI | Delayed-release Capsules | cysteamine bitartrate | 25 mg and 75 mg | 203389 | 1 | 2020-05-11 |
US Patents and Regulatory Information for PROCYSBI
PROCYSBI is protected by twelve US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PROCYSBI is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PROCYSBI
Methods for storing cysteamine formulations and related methods of treatment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods for storing cysteamine formulations and related methods of treatment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods for storing Cysteamine formulations and related methods of treatment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cystamine, cysteamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Delayed release cysteamine bead formulation, and methods of making and using same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Delayed release cysteamine bead formulation, and methods of making and using same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting PROCYSBI
TREATMENT OF NEPHROPATHIC CYSTINOSIS IN PEDIATRIC PATIENTS 1 YEAR OF AGE TO LESS THAN 2 YEARS OF AGE
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
FDA HAS NOT RECOGNIZED ORPHAN-DRUG EXCLUSIVITY (ODE) FOR THIS DRUG, BUT IT CONTAINS THE SAME ACTIVE MOIETY OR MOIETIES AS ANOTHER DRUG(S) THAT WAS ELIGIBLE FOR ODE, AND ALSO SHARES ODE-PROTECTED USE(S) OR INDICATION(S) WITH THAT DRUG(S).AN APPLICATION SEEKING APPROVAL FOR THE SAME ACTIVE MOIETY OR MOIETIES, INCLUDING AN ANDA THAT CITES THIS NDA AS ITS BASIS OF SUBMISSION, MAY NOT BE APPROVED FOR SUCH ODE-PROTECTED USE(S) AND INDICATION(S)
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PROCYSBI
When does loss-of-exclusivity occur for PROCYSBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6628
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 14281702
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2015031417
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 14770
Estimated Expiration: ⤷ Try a Trial
Patent: 38644
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 15003662
Estimated Expiration: ⤷ Try a Trial
China
Patent: 5492000
Estimated Expiration: ⤷ Try a Trial
Patent: 0664780
Estimated Expiration: ⤷ Try a Trial
Cuba
Patent: 150178
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1255
Estimated Expiration: ⤷ Try a Trial
Patent: 1690036
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 10491
Estimated Expiration: ⤷ Try a Trial
Patent: 39574
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 18066
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 4823
Estimated Expiration: ⤷ Try a Trial
Patent: 2141
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 68661
Estimated Expiration: ⤷ Try a Trial
Patent: 16523250
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 15017366
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 4517
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 1500177
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 015502783
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201510126Q
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1508783
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2281747
Estimated Expiration: ⤷ Try a Trial
Patent: 2466253
Estimated Expiration: ⤷ Try a Trial
Patent: 160045053
Estimated Expiration: ⤷ Try a Trial
Patent: 210094140
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 49100
Estimated Expiration: ⤷ Try a Trial
Patent: 1534357
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 15000549
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 7833
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PROCYSBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2016523250 | 遅延放出システアミンビーズ処方、ならびにその作製方法および使用方法 | ⤷ Try a Trial |
| Luxembourg | 92389 | ⤷ Try a Trial | |
| South Korea | 20160045053 | 서방성 시스테아민 비드 투약 형태 (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION) | ⤷ Try a Trial |
| Eurasian Patent Organization | 031255 | СОСТАВ С ОТСРОЧЕННЫМ ВЫСВОБОЖДЕНИЕМ, СОДЕРЖАЩИЙ ГРАНУЛЫ ЦИСТЕАМИНА, И СПОСОБЫ ЕГО ПОЛУЧЕНИЯ И ПРИМЕНЕНИЯ (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION AND METHODS FOR THE PREPARATION AND USE THEREOF) | ⤷ Try a Trial |
| Poland | 2535044 | ⤷ Try a Trial | |
| Tunisia | 2015000549 | DELAYED RELEASE CYSTEAMINE BEAD FORMULATION | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROCYSBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1919458 | 194 50001-2014 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: MERKAPTAMIN; FIRST REGISTRATION: EU/1/13/861, 20130906 |
| 1919458 | 2014C/018 | Belgium | ⤷ Try a Trial | PRODUCT NAME: CYSTEAMINE; AUTHORISATION NUMBER AND DATE: EU/1/13/861 20130910 |
| 1919458 | CR 2014 00013 | Denmark | ⤷ Try a Trial | PRODUCT NAME: CYSTEAMIN, HERUNDER MERCAPTAMINBITARTRAT; REG. NO/DATE: EU/1/13/861 20130906 |
| 1919458 | C 2014 012 | Romania | ⤷ Try a Trial | PRODUCT NAME: CISTEAMINABITARTRAT; NATIONAL AUTHORISATION NUMBER: EU/1/13/861; DATE OF NATIONAL AUTHORISATION: 20130906; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/861; DATE OF FIRST AUTHORISATION IN EEA: 20130906 |
| 1919458 | C01919458/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: MERCAPTAMIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67129 16.08.2019 |
| 1919458 | 19/2014 | Austria | ⤷ Try a Trial | PRODUCT NAME: CYSTEAMIN; REGISTRATION NO/DATE: EU/1/13/861/001-002 20130910 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


