PROCYSBI Drug Patent Profile
✉ Email this page to a colleague
When do Procysbi patents expire, and when can generic versions of Procysbi launch?
Procysbi is a drug marketed by Horizon and is included in two NDAs. There are twelve patents protecting this drug and two Paragraph IV challenges.
This drug has sixty-six patent family members in thirty-four countries.
The generic ingredient in PROCYSBI is cysteamine bitartrate. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the cysteamine bitartrate profile page.
DrugPatentWatch® Generic Entry Outlook for Procysbi
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 17, 2034. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PROCYSBI?
- What are the global sales for PROCYSBI?
- What is Average Wholesale Price for PROCYSBI?
Summary for PROCYSBI
| International Patents: | 66 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 25 |
| Clinical Trials: | 1 |
| Patent Applications: | 197 |
| Drug Prices: | Drug price information for PROCYSBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROCYSBI |
| What excipients (inactive ingredients) are in PROCYSBI? | PROCYSBI excipients list |
| DailyMed Link: | PROCYSBI at DailyMed |
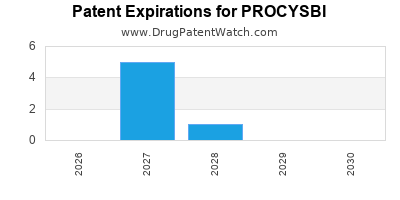

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PROCYSBI
Generic Entry Dates for PROCYSBI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE, DELAYED RELEASE;ORAL |
Generic Entry Dates for PROCYSBI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
GRANULE, DELAYED RELEASE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PROCYSBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Horizon Pharma USA, Inc. | Phase 3 |
| Raptor Pharmaceuticals Inc. | Phase 3 |
Pharmacology for PROCYSBI
| Drug Class | Cystine Depleting Agent |
| Mechanism of Action | Cystine Disulfide Reduction |
Paragraph IV (Patent) Challenges for PROCYSBI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROCYSBI | Delayed-release Granules | cysteamine bitartrate | 75 mg/Packet and 300 mg/Packet | 213491 | 1 | 2021-12-16 |
| PROCYSBI | Delayed-release Capsules | cysteamine bitartrate | 25 mg and 75 mg | 203389 | 1 | 2020-05-11 |
US Patents and Regulatory Information for PROCYSBI
PROCYSBI is protected by twelve US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PROCYSBI is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PROCYSBI
When does loss-of-exclusivity occur for PROCYSBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6628
Estimated Expiration: ⤷ Get Started Free
Patent: 8816
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 14281702
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015031417
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 14770
Estimated Expiration: ⤷ Get Started Free
Patent: 38644
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 15003662
Estimated Expiration: ⤷ Get Started Free
China
Patent: 5492000
Patent: Delayed release cysteamine bead formulation
Estimated Expiration: ⤷ Get Started Free
Patent: 0664780
Patent: 延迟释放型半胱胺珠粒调配物,以及其制备及使用方法 (Delayed release cysteamine bead formulation, and method of manufacture and use thereof)
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 150178
Patent: FORMULACIÓN EN PERLAS DE CISTEAMINA DE LIBERACIÓN RETARDADA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1255
Patent: СОСТАВ С ОТСРОЧЕННЫМ ВЫСВОБОЖДЕНИЕМ, СОДЕРЖАЩИЙ ГРАНУЛЫ ЦИСТЕАМИНА, И СПОСОБЫ ЕГО ПОЛУЧЕНИЯ И ПРИМЕНЕНИЯ (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION AND METHODS FOR THE PREPARATION AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 1690036
Patent: СОСТАВ С ОТСРОЧЕННЫМ ВЫСВОБОЖДЕНИЕМ, СОДЕРЖАЩИЙ ГРАНУЛЫ ЦИСТЕАМИНА, И СПОСОБЫ ЕГО ПОЛУЧЕНИЯ И ПРИМЕНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 10491
Patent: PRÉPARATION DE BILLES DE CYSTÉAMINE À LIBÉRATION RETARDÉE (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 39574
Patent: PRÉPARATION DE BILLES DE CYSTÉAMINE À LIBÉRATION RETARDÉE (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 18066
Patent: 延遲釋放型半胱胺珠粒調配物 (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4823
Patent: פורמולצית bead של ציסטאמין בעל שחרור מושהה, שיטות להכנתה ולשימוש בה (Delayed release cysteamine bead formulation, and methods of making and using same)
Estimated Expiration: ⤷ Get Started Free
Patent: 2141
Patent: פורמולצית bead של ציסטאמין בעל שחרור מושהה, שיטות להכנתה ולשימוש בה (Delayed release cysteamine bead formulation, and methods of making and using same)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 68661
Estimated Expiration: ⤷ Get Started Free
Patent: 16523250
Patent: 遅延放出システアミンビーズ処方、ならびにその作製方法および使用方法
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 5377
Patent: FORMULACIÓN DE PERLAS DE CISTEAMINA DE LIBERACIÓN RETARDADA. (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 15017366
Patent: FORMULACION DE PERLAS DE CISTEAMINA DE LIBERACION RETARDADA. (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4517
Patent: Delayed release cysteamine bead formulation
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1500177
Patent: FORMULACIÓN EN PERLAS DE CISTEAMINA DE LIBERACIÓN RETARDADA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 015502783
Patent: DELAYED RELEASE CYSTEAMINE BEAD FORMULATION
Estimated Expiration: ⤷ Get Started Free
Patent: 020552266
Patent: DELAYED RELEASE CYSTEAMINE BEAD FORMULATION
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201510126Q
Patent: DELAYED RELEASE CYSTEAMINE BEAD FORMULATION
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1508783
Patent: DELAYED RELEASE CYSTEAMINE BEAD FORMULATION
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2281747
Estimated Expiration: ⤷ Get Started Free
Patent: 2466253
Estimated Expiration: ⤷ Get Started Free
Patent: 160045053
Patent: 서방성 시스테아민 비드 투약 형태 (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 210094140
Patent: 서방성 시스테아민 비드 투약 형태 (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 49100
Estimated Expiration: ⤷ Get Started Free
Patent: 1534357
Patent: Delayed release cysteamine bead formulation, and methods of making and using same
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 15000549
Patent: DELAYED RELEASE CYSTEAMINE BEAD FORMULATION
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 7833
Patent: СКЛАД З ВІДСТРОЧЕНИМ ВИВІЛЬНЕННЯМ, ЩО МІСТИТЬ ГРАНУЛИ ЦИСТЕАМІНУ, І СПОСОБИ ЙОГО ОДЕРЖАННЯ ТА ЗАСТОСУВАННЯ (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PROCYSBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2535044 | ⤷ Get Started Free | |
| Mexico | 2008009647 | CISTEAMINA RECUBIERTA ENTÉRICAMENTE, CISTAMINA Y DERIVADOS DE ELLA. (ENTERICALLY COATED CYSTEAMINE, CYSTAMINE AND DERIVATIVES THEREOF.) | ⤷ Get Started Free |
| Japan | 2016523250 | 遅延放出システアミンビーズ処方、ならびにその作製方法および使用方法 | ⤷ Get Started Free |
| Hong Kong | 1218066 | 延遲釋放型半胱胺珠粒調配物 (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION) | ⤷ Get Started Free |
| South Korea | 102466253 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROCYSBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1919458 | C300649 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: CYSTEAMINE; REGISTRATION NO/DATE: EU/1/13/861/001-002 20130906 |
| 1919458 | 2014C/018 | Belgium | ⤷ Get Started Free | PRODUCT NAME: CYSTEAMINE; AUTHORISATION NUMBER AND DATE: EU/1/13/861 20130910 |
| 1919458 | 19/2014 | Austria | ⤷ Get Started Free | PRODUCT NAME: CYSTEAMIN; REGISTRATION NO/DATE: EU/1/13/861/001-002 20130910 |
| 1919458 | C 2014 012 | Romania | ⤷ Get Started Free | PRODUCT NAME: CISTEAMINABITARTRAT; NATIONAL AUTHORISATION NUMBER: EU/1/13/861; DATE OF NATIONAL AUTHORISATION: 20130906; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/861; DATE OF FIRST AUTHORISATION IN EEA: 20130906 |
| 1919458 | CR 2014 00013 | Denmark | ⤷ Get Started Free | PRODUCT NAME: CYSTEAMIN, HERUNDER MERCAPTAMINBITARTRAT; REG. NO/DATE: EU/1/13/861 20130906 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for PROCYSBI: A Comprehensive Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
