PROCYSBI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Procysbi, and what generic alternatives are available?
Procysbi is a drug marketed by Horizon and is included in two NDAs. There are twelve patents protecting this drug and two Paragraph IV challenges.
This drug has sixty-two patent family members in thirty-four countries.
The generic ingredient in PROCYSBI is cysteamine bitartrate. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the cysteamine bitartrate profile page.
DrugPatentWatch® Generic Entry Outlook for Procysbi
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 17, 2034. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for PROCYSBI
| International Patents: | 62 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 13 |
| Clinical Trials: | 1 |
| Patent Applications: | 15 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PROCYSBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROCYSBI |
| What excipients (inactive ingredients) are in PROCYSBI? | PROCYSBI excipients list |
| DailyMed Link: | PROCYSBI at DailyMed |
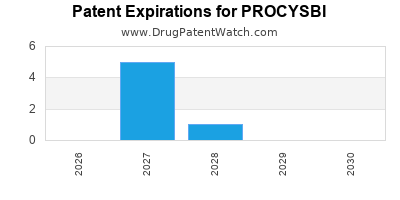

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PROCYSBI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PROCYSBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Raptor Pharmaceuticals Inc. | Phase 3 |
| Horizon Pharma USA, Inc. | Phase 3 |
Pharmacology for PROCYSBI
| Drug Class | Cystine Depleting Agent |
| Mechanism of Action | Cystine Disulfide Reduction |
Paragraph IV (Patent) Challenges for PROCYSBI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROCYSBI | Delayed-release Granules | cysteamine bitartrate | 75 mg/Packet and 300 mg/Packet | 213491 | 1 | 2021-12-16 |
| PROCYSBI | Delayed-release Capsules | cysteamine bitartrate | 25 mg and 75 mg | 203389 | 1 | 2020-05-11 |
US Patents and Regulatory Information for PROCYSBI
PROCYSBI is protected by twelve US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PROCYSBI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PROCYSBI
Methods for storing cysteamine formulations and related methods of treatment
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for storing cysteamine formulations and related methods of treatment
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for storing Cysteamine formulations and related methods of treatment
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cystamine, cysteamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Delayed release cysteamine bead formulation, and methods of making and using same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Delayed release cysteamine bead formulation, and methods of making and using same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Enterically coated cysteamine, cystamine and derivatives thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting PROCYSBI
TREATMENT OF NEPHROPATHIC CYSTINOSIS IN PEDIATRIC PATIENTS 1 YEAR OF AGE TO LESS THAN 2 YEARS OF AGE
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
FDA HAS NOT RECOGNIZED ORPHAN-DRUG EXCLUSIVITY (ODE) FOR THIS DRUG, BUT IT CONTAINS THE SAME ACTIVE MOIETY OR MOIETIES AS ANOTHER DRUG(S) THAT WAS ELIGIBLE FOR ODE, AND ALSO SHARES ODE-PROTECTED USE(S) OR INDICATION(S) WITH THAT DRUG(S).AN APPLICATION SEEKING APPROVAL FOR THE SAME ACTIVE MOIETY OR MOIETIES, INCLUDING AN ANDA THAT CITES THIS NDA AS ITS BASIS OF SUBMISSION, MAY NOT BE APPROVED FOR SUCH ODE-PROTECTED USE(S) AND INDICATION(S)
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-001 | Feb 14, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PROCYSBI
When does loss-of-exclusivity occur for PROCYSBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6628
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 14281702
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2015031417
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 14770
Estimated Expiration: ⤷ Sign Up
Patent: 38644
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 15003662
Estimated Expiration: ⤷ Sign Up
China
Patent: 5492000
Estimated Expiration: ⤷ Sign Up
Patent: 0664780
Estimated Expiration: ⤷ Sign Up
Cuba
Patent: 150178
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1255
Estimated Expiration: ⤷ Sign Up
Patent: 1690036
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 10491
Estimated Expiration: ⤷ Sign Up
Patent: 39574
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 18066
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 4823
Estimated Expiration: ⤷ Sign Up
Patent: 2141
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 68661
Estimated Expiration: ⤷ Sign Up
Patent: 16523250
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 15017366
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 4517
Estimated Expiration: ⤷ Sign Up
Nicaragua
Patent: 1500177
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 015502783
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201510126Q
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1508783
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2281747
Estimated Expiration: ⤷ Sign Up
Patent: 2466253
Estimated Expiration: ⤷ Sign Up
Patent: 160045053
Estimated Expiration: ⤷ Sign Up
Patent: 210094140
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 49100
Estimated Expiration: ⤷ Sign Up
Patent: 1534357
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 15000549
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 7833
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PROCYSBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Eurasian Patent Organization | 201690036 | СОСТАВ С ОТСРОЧЕННЫМ ВЫСВОБОЖДЕНИЕМ, СОДЕРЖАЩИЙ ГРАНУЛЫ ЦИСТЕАМИНА, И СПОСОБЫ ЕГО ПОЛУЧЕНИЯ И ПРИМЕНЕНИЯ | ⤷ Sign Up |
| European Patent Office | 2535044 | Cysteamine bitartrate et cystamine a enrobage entérique (Enterically coated cysteamine bitartrate and cystamine) | ⤷ Sign Up |
| European Patent Office | 3659588 | CYSTÉAMINE ENTÉROSOLUBLE ET SELS ASSOCIÉS (ENTERICALLY COATED CYSTEAMINE AND SALTS THEREOF) | ⤷ Sign Up |
| European Patent Office | 3010491 | PRÉPARATION DE BILLES DE CYSTÉAMINE À LIBÉRATION RETARDÉE (DELAYED RELEASE CYSTEAMINE BEAD FORMULATION) | ⤷ Sign Up |
| Taiwan | I649100 | ⤷ Sign Up | |
| South Africa | 201508783 | DELAYED RELEASE CYSTEAMINE BEAD FORMULATION | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROCYSBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1919458 | C 2014 012 | Romania | ⤷ Sign Up | PRODUCT NAME: CISTEAMINABITARTRAT; NATIONAL AUTHORISATION NUMBER: EU/1/13/861; DATE OF NATIONAL AUTHORISATION: 20130906; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/861; DATE OF FIRST AUTHORISATION IN EEA: 20130906 |
| 1919458 | 122014000023 | Germany | ⤷ Sign Up | PRODUCT NAME: CYSTEAMIN ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/13/861 20130906 |
| 1919458 | 2014C/018 | Belgium | ⤷ Sign Up | PRODUCT NAME: CYSTEAMINE; AUTHORISATION NUMBER AND DATE: EU/1/13/861 20130910 |
| 1919458 | C01919458/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: MERCAPTAMIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67129 16.08.2019 |
| 1919458 | CR 2014 00013 | Denmark | ⤷ Sign Up | PRODUCT NAME: CYSTEAMIN, HERUNDER MERCAPTAMINBITARTRAT; REG. NO/DATE: EU/1/13/861 20130906 |
| 1919458 | 19/2014 | Austria | ⤷ Sign Up | PRODUCT NAME: CYSTEAMIN; REGISTRATION NO/DATE: EU/1/13/861/001-002 20130910 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


