PREZCOBIX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Prezcobix, and when can generic versions of Prezcobix launch?
Prezcobix is a drug marketed by Janssen Prods and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and forty-three patent family members in forty countries.
The generic ingredient in PREZCOBIX is cobicistat; darunavir. There are five drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the cobicistat; darunavir profile page.
DrugPatentWatch® Generic Entry Outlook for Prezcobix
Prezcobix was eligible for patent challenges on August 27, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 6, 2032. This may change due to patent challenges or generic licensing.
There have been thirteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for PREZCOBIX
| International Patents: | 343 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 4 |
| Patent Applications: | 2 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PREZCOBIX |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PREZCOBIX |
| What excipients (inactive ingredients) are in PREZCOBIX? | PREZCOBIX excipients list |
| DailyMed Link: | PREZCOBIX at DailyMed |
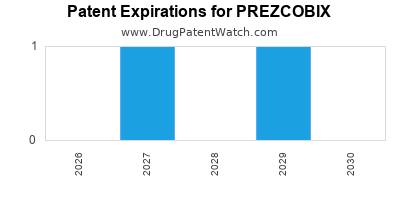
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PREZCOBIX
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PREZCOBIX
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Janssen Pharmaceutical K.K. | Phase 4 |
| University of Colorado, Denver | Phase 2/Phase 3 |
| Janssen Scientific Affairs, LLC | Phase 2/Phase 3 |
Pharmacology for PREZCOBIX
Anatomical Therapeutic Chemical (ATC) Classes for PREZCOBIX
Paragraph IV (Patent) Challenges for PREZCOBIX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PREZCOBIX | Tablets | cobicistat; darunavir | 800 mg/150 mg | 205395 | 1 | 2020-07-24 |
US Patents and Regulatory Information for PREZCOBIX
PREZCOBIX is protected by five US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PREZCOBIX is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PREZCOBIX
Use of solid carrier particles to improve the processability of a pharmaceutical agent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pseudopolymorphic forms of a HIV protease inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modulators of pharmacokinetic properties of therapeutics
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION USING A COMPOSITION CONTAINING A PHARMACOKINETIC ENHANCER THAT INHIBITS CYTOCHROME P450 MONOOXYGENASE
Modulators of pharmacokinetic properties of therapeutics
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF HIV INFECTION IN ADULTS AND PEDIATRIC PATIENTS WEIGHING AT LEAST 40KG USING A COMPOSITION CONTAINING A PHARMACOKINETIC ENHANCER THAT INHIBITS CYTOCHROME P450 MONOOXYGENASE
Pseudopolymorphic forms of a HIV protease inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PREZCOBIX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Prods | PREZCOBIX | cobicistat; darunavir | TABLET;ORAL | 205395-001 | Jan 29, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for PREZCOBIX
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International N.V. | Rezolsta | darunavir, cobicistat | EMEA/H/C/002819 Rezolsta, is indicated in combination with other antiretroviral medicinal products for the treatment of human immunodeficiency virus 1 (HIV 1) infection in adults aged 18 years or older.Genotypic testing should guide the use of Rezolsta. |
Authorised | no | no | no | 2014-11-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PREZCOBIX
When does loss-of-exclusivity occur for PREZCOBIX?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 89
Estimated Expiration: ⤷ Sign Up
Patent: 50
Estimated Expiration: ⤷ Sign Up
Argentina
Patent: 5369
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 09242451
Estimated Expiration: ⤷ Sign Up
Patent: 10210598
Estimated Expiration: ⤷ Sign Up
Patent: 14221210
Estimated Expiration: ⤷ Sign Up
Patent: 15200637
Estimated Expiration: ⤷ Sign Up
Patent: 16250470
Estimated Expiration: ⤷ Sign Up
Patent: 17201473
Estimated Expiration: ⤷ Sign Up
Patent: 18267573
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0911871
Estimated Expiration: ⤷ Sign Up
Patent: 1008664
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 20856
Estimated Expiration: ⤷ Sign Up
Patent: 50521
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 11001885
Estimated Expiration: ⤷ Sign Up
China
Patent: 2123700
Estimated Expiration: ⤷ Sign Up
Patent: 2307573
Estimated Expiration: ⤷ Sign Up
Patent: 3479584
Estimated Expiration: ⤷ Sign Up
Patent: 4940937
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 21225
Estimated Expiration: ⤷ Sign Up
Patent: 00187
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0151009
Estimated Expiration: ⤷ Sign Up
Patent: 0151357
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 16852
Estimated Expiration: ⤷ Sign Up
Patent: 17067
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 96633
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 10010636
Estimated Expiration: ⤷ Sign Up
Patent: 11011307
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1313
Estimated Expiration: ⤷ Sign Up
Patent: 2950
Estimated Expiration: ⤷ Sign Up
Patent: 0123
Estimated Expiration: ⤷ Sign Up
Patent: 1071173
Estimated Expiration: ⤷ Sign Up
Patent: 1190125
Estimated Expiration: ⤷ Sign Up
Patent: 1491658
Estimated Expiration: ⤷ Sign Up
Patent: 1591353
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 96633
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
Patent: 06032
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 53670
Estimated Expiration: ⤷ Sign Up
Patent: 64737
Estimated Expiration: ⤷ Sign Up
Patent: 15679
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 25822
Estimated Expiration: ⤷ Sign Up
Patent: 26380
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 8614
Estimated Expiration: ⤷ Sign Up
Patent: 4227
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 11242
Estimated Expiration: ⤷ Sign Up
Patent: 22213
Estimated Expiration: ⤷ Sign Up
Patent: 11927
Estimated Expiration: ⤷ Sign Up
Patent: 25171
Estimated Expiration: ⤷ Sign Up
Patent: 11522790
Estimated Expiration: ⤷ Sign Up
Patent: 12517432
Estimated Expiration: ⤷ Sign Up
Patent: 14012741
Estimated Expiration: ⤷ Sign Up
Patent: 14221845
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 2377
Estimated Expiration: ⤷ Sign Up
Patent: 10011963
Estimated Expiration: ⤷ Sign Up
Patent: 11008289
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 8978
Estimated Expiration: ⤷ Sign Up
Patent: 4214
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 110994
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 96633
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 96633
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
San Marino
Patent: 01500266
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 3544
Estimated Expiration: ⤷ Sign Up
Patent: 0618
Estimated Expiration: ⤷ Sign Up
Patent: 14007744
Estimated Expiration: ⤷ Sign Up
Patent: 201609006W
Estimated Expiration: ⤷ Sign Up
Patent: 201706215U
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 96633
Estimated Expiration: ⤷ Sign Up
Patent: 93485
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1008007
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1645759
Estimated Expiration: ⤷ Sign Up
Patent: 1659971
Estimated Expiration: ⤷ Sign Up
Patent: 1738325
Estimated Expiration: ⤷ Sign Up
Patent: 1784647
Estimated Expiration: ⤷ Sign Up
Patent: 110015581
Estimated Expiration: ⤷ Sign Up
Patent: 110122729
Estimated Expiration: ⤷ Sign Up
Patent: 160093100
Estimated Expiration: ⤷ Sign Up
Patent: 160114728
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 48886
Estimated Expiration: ⤷ Sign Up
Patent: 53897
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 44367
Estimated Expiration: ⤷ Sign Up
Patent: 1040142
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 1193
Estimated Expiration: ⤷ Sign Up
Patent: 3224
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 424
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PREZCOBIX around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2767539 | ⤷ Sign Up | |
| Spain | 2796275 | ⤷ Sign Up | |
| Australia | 4828199 | ⤷ Sign Up | |
| Australia | 2008218186 | Modulators of pharmacokinetic properties of therapeutics | ⤷ Sign Up |
| Lithuania | 3150586 | ⤷ Sign Up | |
| Cyprus | 1116852 | ⤷ Sign Up | |
| Eurasian Patent Organization | 022950 | ПРИМЕНЕНИЕ ЧАСТИЦ НОСИТЕЛЯ ДИОКСИДА КРЕМНИЯ ДЛЯ УЛУЧШЕНИЯ ТЕХНОЛОГИЧЕСКИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧЕСКОГО АГЕНТА (USE OF SILICON DIOXIDE CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PREZCOBIX
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2487163 | CA 2017 00003 | Denmark | ⤷ Sign Up | PRODUCT NAME: COBICISTAT ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF OG ATAZANAVIR ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, ISAER ATAZANAVIRSULFAT; REG. NO/DATE: EU/1/15/1025/001-002 20150715 |
| 2049506 | 1590060-8 | Sweden | ⤷ Sign Up | PRODUCT NAME: COBICISTAT OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF |
| 2487166 | 2016/061 | Ireland | ⤷ Sign Up | PRODUCT NAME: COBICISTAT OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND TENOFOVIR ALAFENAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR TENOFOVIR ALAFENAMIDE FUMARATE; REGISTRATION NO/DATE: EU/1/15/1061 20151119 |
| 2049506 | 15C0078 | France | ⤷ Sign Up | PRODUCT NAME: COBICISTAT OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE COBICISTAT; REGISTRATION NO/DATE: EU/1/13/830/001-002 20130527 |
| 2049506 | 300780 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COBICISTAT OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/13/830/001-002 20130527 |
| 2049506 | 122015000092 | Germany | ⤷ Sign Up | PRODUCT NAME: COBICISTAT ODER EIN PHARMAZEUTISCH UNBEDENKLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/13/830/001-002 20130524 |
| 2049506 | 2015C/058 | Belgium | ⤷ Sign Up | PRODUCT NAME: COBICISTAT; AUTHORISATION NUMBER AND DATE: EU/1/13/830/001 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


