PRADAXA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Pradaxa, and what generic alternatives are available?
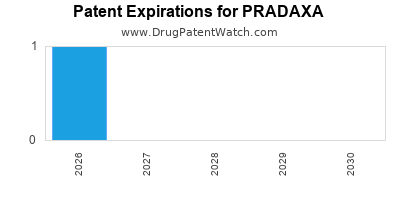
Pradaxa is a drug marketed by Boehringer Ingelheim and is included in two NDAs. There are three patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and forty-two patent family members in forty-four countries.
The generic ingredient in PRADAXA is dabigatran etexilate mesylate. There are twenty-six drug master file entries for this compound. Eight suppliers are listed for this compound. Additional details are available on the dabigatran etexilate mesylate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Pradaxa
A generic version of PRADAXA was approved as dabigatran etexilate mesylate by ALKEM LABS LTD on March 11th, 2020.
Summary for PRADAXA
| International Patents: | 142 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 12 |
| Clinical Trials: | 55 |
| Patent Applications: | 96 |
| Formulation / Manufacturing: | see details |
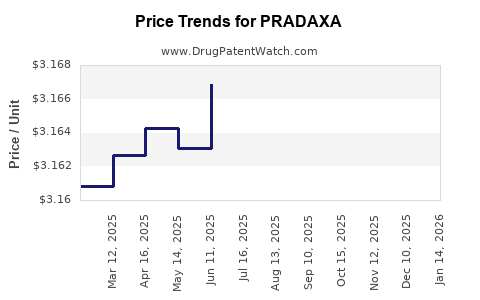
| Drug Prices: | Drug price information for PRADAXA |
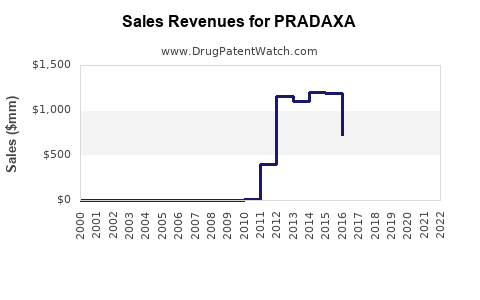
| Drug Sales Revenues: | Drug sales revenues for PRADAXA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PRADAXA |
| What excipients (inactive ingredients) are in PRADAXA? | PRADAXA excipients list |
| DailyMed Link: | PRADAXA at DailyMed |
Recent Clinical Trials for PRADAXA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Pfizer | Phase 1 |
| Laval University | Phase 2 |
| Bayer | Phase 1 |
Pharmacology for PRADAXA
| Drug Class | Direct Thrombin Inhibitor |
| Mechanism of Action | Thrombin Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for PRADAXA
Paragraph IV (Patent) Challenges for PRADAXA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PRADAXA | Capsules | dabigatran etexilate mesylate | eq. to 110 mg base | 022512 | 2 | 2015-12-15 |
| PRADAXA | Capsules | dabigatran etexilate mesylate | eq. to 75 mg base and 150 mg base | 022512 | 17 | 2014-10-20 |
US Patents and Regulatory Information for PRADAXA
PRADAXA is protected by three US patents and three FDA Regulatory Exclusivities.
Patents protecting PRADAXA
Film container
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
3-[(2-{[4-(hexyloxycarbonylaminoiminomethyl) phenylamino]methyl}-1-methyl-1H-benzimidazol-5-carbonyl)pyridin-2-ylamino- ]propionic acid ethylester methansulfonate and its use as a medicament
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of using antibodies during anticoagulant therapy of dabigatran and/or related compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting PRADAXA
TREATMENT OF VENOUS THROMBOEMBOLIC EVENTS (VTE) IN PEDIATRIC PATIENTS 8 TO LESS THAN 18 YEARS OF AGE WHO HAVE BEEN TREATED WITH A PARENTERAL ANTICOAGULANT FOR AT LEAST 5 DAYS AND TO REDUCE THE RISK OF RECURRENCE OF VTE IN PEDIATRIC PATIENTS 8 TO LESS THAN 18 YEARS OF AGE WHO HAVE BEEN PREVIOUSLY TREATED
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
NEW PRODUCT
Exclusivity Expiration: ⤷ Try a Trial
International Patents for PRADAXA
See the table below for patents covering PRADAXA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Taiwan | 588047 | ⤷ Try a Trial | |
| Slovenia | 2525812 | ⤷ Try a Trial | |
| Norway | 332209 | ⤷ Try a Trial | |
| Norway | 2008012 | ⤷ Try a Trial | |
| Australia | 2005318231 | Film container | ⤷ Try a Trial |
| European Patent Office | 0966454 | HETEROCYCLES BICYCLIQUES DISUBSTITUES, PRODUCTION ET UTILISATION COMME MEDICAMENTS (DISUBSTITUTED BICYCLIC HETEROCYCLES, THEIR PRODUCTION AND USE AS MEDICAMENTS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PRADAXA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1870100 | 132012902065811 | Italy | ⤷ Try a Trial | PRODUCT NAME: DABIGATRAN ETEXILATO MESILATO(PRADAXA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/08/442/001-2-3-4-5-6-7-8, 20080318 |
| 1870100 | CA 2012 00027 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DABIGATRAN-ETEXILAT (SOM MESILAT); REG. NO/DATE: EU/1/08/442/001-002 20080318 |
| 2525812 | 627 | Finland | ⤷ Try a Trial | |
| 2525812 | C201730027 | Spain | ⤷ Try a Trial | PRODUCT NAME: IDARUCIZUMAB; NATIONAL AUTHORISATION NUMBER: EU/1/15/1056; DATE OF AUTHORISATION: 20151120; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1056; DATE OF FIRST AUTHORISATION IN EEA: 20151120 |
| 2525812 | PA2017021,C2525812 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IDARUCIZUMABAS; REGISTRATION NO/DATE: EU/1/15/1056/001 20151120 |
| 2525812 | 1790034-1 | Sweden | ⤷ Try a Trial | PRODUCT NAME: IDARUCIZUMAB GODKAENNANDE EU/1/15/1056 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


