POMALYST Drug Patent Profile
✉ Email this page to a colleague
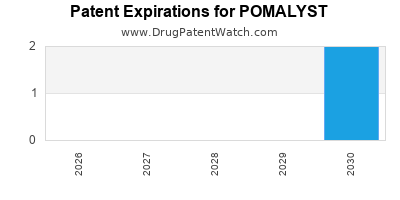
When do Pomalyst patents expire, and when can generic versions of Pomalyst launch?
Pomalyst is a drug marketed by Bristol and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and fifty-seven patent family members in forty-eight countries.
The generic ingredient in POMALYST is pomalidomide. There are eleven drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the pomalidomide profile page.
DrugPatentWatch® Generic Entry Outlook for Pomalyst
Pomalyst was eligible for patent challenges on February 8, 2017.
There have been twenty-eight patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (pomalidomide), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for POMALYST
| International Patents: | 357 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 147 |
| Clinical Trials: | 82 |
| Patent Applications: | 2,650 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for POMALYST |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for POMALYST |
| What excipients (inactive ingredients) are in POMALYST? | POMALYST excipients list |
| DailyMed Link: | POMALYST at DailyMed |

Recent Clinical Trials for POMALYST
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Chicago | Phase 2 |
| Regeneron Pharmaceuticals | Phase 3 |
| Bristol-Myers Squibb | Phase 3 |
Pharmacology for POMALYST
| Drug Class | Thalidomide Analog |
Anatomical Therapeutic Chemical (ATC) Classes for POMALYST
Paragraph IV (Patent) Challenges for POMALYST
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| POMALYST | Capsules | pomalidomide | 1 mg, 2 mg, 3 mg and 4 mg | 204026 | 6 | 2017-02-08 |
US Patents and Regulatory Information for POMALYST
POMALYST is protected by four US patents and five FDA Regulatory Exclusivities.
Patents protecting POMALYST
Formulations of 4-amino-2-(2,6-dioxopiperidine-3-yl)isoindoline-1,3-dione
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for treating multiple myeloma using 4-(amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Formulations of 4-amino-2-(2,6-dioxopiperidine-3-yl)isoindoline-1,3-dione
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Formulations of 4-amino-2-(2,6-dioxopiperidine-3-yl)isoindoline-1,3-dione
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting POMALYST
ADDITIONAL CLINICAL TRIAL INFORMATION ADDED TO PEDIATRIC USE SUBSECTION
Exclusivity Expiration: ⤷ Sign Up
FOR THE TREATMENT OF KAPOSI SARCOMA (KS) IN ADULT PATIENTS WHO ARE HIV-NEGATIVE
Exclusivity Expiration: ⤷ Sign Up
INDICATED FOR THE TREATMENT OF ADULT PATIENTS WITH AIDS-RELATED KAPOSI SARCOMA (KS) AFTER FAILURE OF HIGHLY ACTIVE ANTIRETROVIRAL THERAPY (HAART)
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-004 | Feb 8, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-003 | Feb 8, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-004 | Feb 8, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for POMALYST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-002 | Feb 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-002 | Feb 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Bristol | POMALYST | pomalidomide | CAPSULE;ORAL | 204026-002 | Feb 8, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for POMALYST
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bristol-Myers Squibb Pharma EEIG | Imnovid (previously Pomalidomide Celgene) | pomalidomide | EMEA/H/C/002682 Imnovid in combination with bortezomib and dexamethasone is indicated in the treatment of adult patients with multiple myeloma who have received at least one prior treatment regimen including lenalidomide.Imnovid in combination with dexamethasone is indicated in the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy. |
Authorised | no | no | no | 2013-08-05 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for POMALYST
See the table below for patents covering POMALYST around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 195916 | ⤷ Sign Up | |
| South Africa | 200803700 | Methods using 3-(4-amino-1oxo-1,3-dihydro-isoindol-2-yl)-piperdine-2,6-dione for treatment of certain leukemias | ⤷ Sign Up |
| Hong Kong | 1202531 | -氨基- -氧代- 二氫-異吲哚- -基 -哌啶- -二酮的水合物 (HYDRATES OF 3-(4-AMINO-1-OXO-1,3 DIHYDRO-ISOINDOL-2-YL)-PIPERIDINE- 2,6-DIONE 3-(4--1--13 --2-)--2,6-) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for POMALYST
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0925294 | C00925294/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: LENALIDOMIDUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 57712 29.08.2007 |
| 0925294 | 07C0056 | France | ⤷ Sign Up | PRODUCT NAME: LENALIDOMIDE; REGISTRATION NO/DATE: EU/1/07/391/001-004 20070618 |
| 0925294 | 91359 | Luxembourg | ⤷ Sign Up | 91359, EXPIRES: 20220614 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


