Last updated: November 19, 2025
Introduction
Pomalidomide, marketed under the brand name POMALYST, is a credited therapeutic agent primarily used for multiple myeloma (MM), particularly in relapsed and refractory cases. Since its approval by the U.S. Food and Drug Administration (FDA) in 2013, POMALYST has established itself as a vital component within hematologic oncology, driven by unique pharmacological properties and strategic market positioning. This analysis explores the evolving market dynamics, competitive landscape, regulatory influences, and financial outlook shaping POMALYST’s trajectory.
Pharmacological Profile and Clinical Positioning
Pomalidomide belongs to the immunomodulatory drug (IMiD) class, akin to thalidomide and lenalidomide, but with enhanced potency against multiple myeloma cells. It exerts anti-inflammatory, immunostimulatory, and anti-angiogenic effects, making it effective in patients refractory to prior therapies, including lenalidomide and bortezomib[^1].
Its clinical use is largely confined to relapsed/refractory multiple myeloma (RRMM), often following failure of previous lines of treatment. The drug’s efficacy, combined with manageable side-effect profiles, sustains demand within oncology markets, especially among heavily pretreated patients.
Market Landscape and Drivers
1. Growing Incidence of Multiple Myeloma
Globally, the incidence of multiple myeloma is rising due to aging populations and improved diagnostics. The American Cancer Society estimates approximately 34,000 new cases annually in the U.S. alone, with similar trends nationally and globally[^2]. This increasing patient pool drives demand for therapeutics like POMALYST.
2. Treatment Paradigm Shifts
The therapeutic landscape for MM has evolved significantly with the advent of novel agents, including proteasome inhibitors, monoclonal antibodies, and IMiDs. POMALYST occupies a critical niche in salvage therapy, particularly in heavily pretreated populations[^3].
Furthermore, combination regimens involving POMALYST (e.g., with dexamethasone, daratumumab) demonstrate enhanced efficacy and are expanding its use, bolstering market growth.
3. Evolving Resistance Patterns and Unmet Needs
Resistance to existing therapies prompts clinicians to seek alternative options like POMALYST. Its efficacy in patients refractory to lenalidomide and bortezomib underpins its value, sustaining its clinical and commercial relevance.
4. Competitive Dynamics
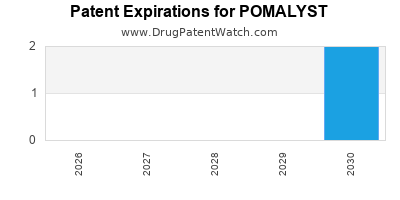
While POMALYST enjoys market exclusivity until 2027 in the U.S. owing to patent protection, its position faces competition from emerging agents, biosimilars, and next-generation therapies. Notably, agents like selinexor and CAR-T cell therapies are beginning to redefine RRMM treatment algorithms[^4].
5. Regulatory and Reimbursement Environment
Reimbursement policies and potential price negotiations influence market access, particularly in key regions like the U.S., Europe, and Asia. The pricing of POMALYST, justified by its clinical benefits, remains a key driver of revenue.
Financial Trajectory and Revenue Streams
1. Historical and Projected Sales Data
Since its launch, POMALYST has experienced robust revenue growth attributable to its first-mover advantage and clinical efficacy. For example, in 2020, the drug generated approximately $1 billion globally, with continued growth projected as adoption expands[^5].
The COVID-19 pandemic temporarily disrupted supply chains and patient access, but recovery has been swift due to the ongoing necessity of MM therapies.
2. Market Penetration and Geographic Expansion
Initially dominated by the U.S., POMALYST’s sales are increasingly driven by European and Asian markets, aided by regulatory approvals and localized reimbursement strategies[^6].
The rollout of combination treatments and inclusion in clinical guidelines enhances its adoption, supporting sustained revenue streams.
3. Patent Protection and Generic Competition
Patent exclusivity extends until 2027 in the U.S., underpinning revenue stability. Post-expiry, biosimilars and generics may emerge, exerting downward pressure on pricing and revenues.
Strategic lifecycle management, including new formulation development and expanding indications, aims to prolong market dominance.
4. Investment in Clinical Development
Biogen and Celgene (now part of Bristol-Myers Squibb following acquisition of Celgene in 2019) continue to invest in trials exploring POMALYST in combination with novel agents and indications like solid tumors, which could unlock additional revenue sources[^7].
Regulatory and Market Challenges
1. Safety and Side-effect Profile
Adverse effects such as neuropathy, myelosuppression, and thromboembolic events necessitate vigilant management, impacting prescribing patterns and reimbursement negotiations.
2. Competition and Drug Discontinuation Risks
Emergence of superior therapies may limit POMALYST’s market share, necessitating innovation and combination regimen optimization.
3. Pricing and Access Pressures
Healthcare institutions and payers seek cost containment, potentially threatening profitability through formulary restrictions or price negotiations.
Future Outlook and Strategies
1. Expansion into New Indications
Investigations into POMALYST's efficacy in other hematologic malignancies and solid tumors could diversify revenue streams.
2. Combination Regimens and Personalized Therapies
Developing tailored combination therapies could enhance response rates and extend market relevance. Notable ongoing trials involve POMALYST with monoclonal antibodies and novel agents.
3. Geographic and Demographic Expansion
Growing acceptance in emerging markets, supported by regulatory approvals, will likely accelerate revenue growth. Tailoring pricing strategies to regional economic contexts remains critical.
4. Lifecycle Initiatives
Patent protections provide a window for lifecycle extension via new formulations, dosing schedules, and approved uses, preserving market share amid competitive pressures.
Key Takeaways
- POMALYST remains a cornerstone for relapsed/refractory multiple myeloma, with a growing global market driven by disease prevalence and evolving treatment paradigms.
- Its financial trajectory aligns with expanding indications, combination regimens, and geographic penetration, supported by patent exclusivity until 2027.
- Competitive threats from emerging therapies and biosimilars underscore the importance of ongoing clinical innovation and strategic lifecycle management.
- Regulatory, safety, and reimbursement factors significantly influence market access and profitability, necessitating proactive stakeholder engagement.
- Future growth will depend on successful expansion into new indications, combination strategies, and adaptation to regional healthcare dynamics.
FAQs
1. What distinguishes POMALYST from other immunomodulatory drugs?
POMALYST exhibits greater potency and efficacy in patients refractory to lenalidomide, with a unique mechanism of action that enhances immune modulation and anti-angiogenesis. Its ability to overcome specific resistance patterns differentiates it within the IMiD class.
2. When is POMALYST expected to face generic competition?
Patent protection in the U.S. expires in 2027, after which biosimilars and generic versions are anticipated, potentially impacting pricing and market share.
3. Are there ongoing efforts to expand POMALYST’s approved uses?
Yes. Clinical trials are evaluating its efficacy in other hematologic cancers, such as mantle cell lymphoma and certain solid tumors, aiming to broaden therapeutic indications.
4. How does POMALYST’s safety profile impact its marketability?
While generally well-tolerated, risks like blood clots, neuropathy, and hematological toxicities require management strategies. These may influence clinician preferences and reimbursement negotiations.
5. What is the outlook for POMALYST’s sales in emerging markets?
As approvals expand and healthcare infrastructure improves, sales in Asia, Latin America, and Africa are expected to grow, contingent upon localized pricing strategies and reimbursement policies.
References
[^1]: Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548-567.
[^2]: American Cancer Society. Key Statistics for Multiple Myeloma. 2022.
[^3]: Kumar SK, et al. Multiple myeloma. Lancet. 2021;397(10272):430-447.
[^4]: Lonial S, et al. Treatment strategies for relapsed/refractory multiple myeloma. Nat Rev Clin Oncol. 2022;19(5):321-339.
[^5]: IQVIA. Pharmaceuticals Data and Market Trends Report, 2021.
[^6]: European Medicines Agency. Pomalidomide review and approval summary, 2013.
[^7]: Bristol-Myers Squibb. Annual Report 2022; Clinical Trials Database.