PLENVU Drug Patent Profile
✉ Email this page to a colleague
When do Plenvu patents expire, and when can generic versions of Plenvu launch?
Plenvu is a drug marketed by Salix and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and fifty-nine patent family members in thirty-seven countries.
The generic ingredient in PLENVU is ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate. There are six drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Plenvu
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for PLENVU
| International Patents: | 159 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 6 |
| Formulation / Manufacturing: | see details |
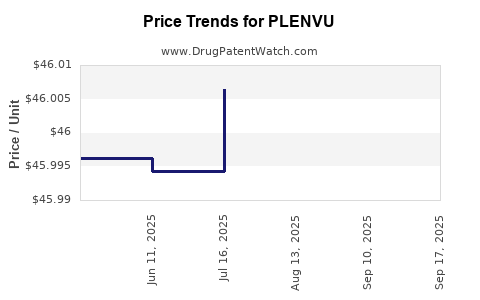
| Drug Prices: | Drug price information for PLENVU |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PLENVU |
| What excipients (inactive ingredients) are in PLENVU? | PLENVU excipients list |
| DailyMed Link: | PLENVU at DailyMed |
Recent Clinical Trials for PLENVU
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Odense University Hospital | Phase 3 |
| Lund University | Phase 3 |
| Region Skane | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for PLENVU
Paragraph IV (Patent) Challenges for PLENVU
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PLENVU | For Oral Solution | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | 140 g, 5.2 g, 2.2.g, 48.11 g, 9 g and 7.54 g per pouch | 209381 | 1 | 2018-12-06 |
US Patents and Regulatory Information for PLENVU
PLENVU is protected by ten US patents.
Patents protecting PLENVU
Compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Colonoscopy-preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Colonoscopy--preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR CLEANSING OF THE COLON IN PREPARATION FOR COLONOSCOPY IN ADULTS
Colon cleansing compositions and methods of use
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR CLEANSING OF THE COLON IN PREPARATION FOR COLONOSCOPY IN ADULTS
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR CLEANSING OF THE COLON IN PREPARATION FOR COLONOSCOPY IN ADULTS
Compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR CLEANSING OF THE COLON IN PREPARATION FOR COLONOSCOPY IN ADULTS
Colonoscopy--preparation
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: FOR CLEANSING OF THE COLON IN PREPARATION FOR COLONOSCOPY IN ADULTS
Compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Salix | PLENVU | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 209381-001 | May 4, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for PLENVU
When does loss-of-exclusivity occur for PLENVU?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2500
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 13314442
Estimated Expiration: ⤷ Sign Up
Patent: 15228962
Estimated Expiration: ⤷ Sign Up
Patent: 18204694
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2015005270
Estimated Expiration: ⤷ Sign Up
Patent: 2016019914
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 84472
Estimated Expiration: ⤷ Sign Up
Patent: 42089
Estimated Expiration: ⤷ Sign Up
Patent: 16204
Estimated Expiration: ⤷ Sign Up
China
Patent: 5007915
Estimated Expiration: ⤷ Sign Up
Patent: 6102738
Estimated Expiration: ⤷ Sign Up
Patent: 5154464
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0191007
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 21668
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 95163
Estimated Expiration: ⤷ Sign Up
Patent: 73248
Estimated Expiration: ⤷ Sign Up
Patent: 77850
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 8866
Estimated Expiration: ⤷ Sign Up
Patent: 7891
Estimated Expiration: ⤷ Sign Up
Patent: 1500320
Estimated Expiration: ⤷ Sign Up
Patent: 1600639
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 95163
Estimated Expiration: ⤷ Sign Up
Patent: 16494
Estimated Expiration: ⤷ Sign Up
Patent: 73248
Estimated Expiration: ⤷ Sign Up
Patent: 77850
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 06604
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 43605
Estimated Expiration: ⤷ Sign Up
India
Patent: 04DEN2015
Estimated Expiration: ⤷ Sign Up
Ireland
Patent: 0130273
Estimated Expiration: ⤷ Sign Up
Patent: 6410
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 7377
Estimated Expiration: ⤷ Sign Up
Patent: 7170
Estimated Expiration: ⤷ Sign Up
Patent: 9024
Estimated Expiration: ⤷ Sign Up
Patent: 4374
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 57675
Estimated Expiration: ⤷ Sign Up
Patent: 55391
Estimated Expiration: ⤷ Sign Up
Patent: 77396
Estimated Expiration: ⤷ Sign Up
Patent: 15527385
Estimated Expiration: ⤷ Sign Up
Patent: 17507965
Estimated Expiration: ⤷ Sign Up
Patent: 20073579
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 95163
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 4758
Estimated Expiration: ⤷ Sign Up
Patent: 8550
Estimated Expiration: ⤷ Sign Up
Patent: 3378
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 9318
Estimated Expiration: ⤷ Sign Up
Patent: 15003031
Estimated Expiration: ⤷ Sign Up
Patent: 16011256
Estimated Expiration: ⤷ Sign Up
Patent: 21005225
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 413
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 11424
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 5499
Estimated Expiration: ⤷ Sign Up
Patent: 3017
Estimated Expiration: ⤷ Sign Up
Patent: 3984
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 95163
Estimated Expiration: ⤷ Sign Up
Patent: 73248
Estimated Expiration: ⤷ Sign Up
Patent: 77850
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 95163
Estimated Expiration: ⤷ Sign Up
Patent: 73248
Estimated Expiration: ⤷ Sign Up
Patent: 77850
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 979
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201702005V
Estimated Expiration: ⤷ Sign Up
Patent: 201807427R
Estimated Expiration: ⤷ Sign Up
Patent: 202112138Q
Estimated Expiration: ⤷ Sign Up
Patent: 201501713X
Estimated Expiration: ⤷ Sign Up
Patent: 201606834Q
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 95163
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1501415
Estimated Expiration: ⤷ Sign Up
Patent: 1603747
Estimated Expiration: ⤷ Sign Up
Patent: 1605592
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1771586
Estimated Expiration: ⤷ Sign Up
Patent: 2234803
Estimated Expiration: ⤷ Sign Up
Patent: 2257207
Estimated Expiration: ⤷ Sign Up
Patent: 150054990
Estimated Expiration: ⤷ Sign Up
Patent: 160130488
Estimated Expiration: ⤷ Sign Up
Patent: 170098980
Estimated Expiration: ⤷ Sign Up
Patent: 190137925
Estimated Expiration: ⤷ Sign Up
Patent: 210063445
Estimated Expiration: ⤷ Sign Up
Patent: 230010844
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 28459
Estimated Expiration: ⤷ Sign Up
Patent: 31412
Estimated Expiration: ⤷ Sign Up
Patent: 09386
Estimated Expiration: ⤷ Sign Up
Patent: 28719
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 87238
Estimated Expiration: ⤷ Sign Up
Patent: 56623
Estimated Expiration: ⤷ Sign Up
Patent: 1416092
Estimated Expiration: ⤷ Sign Up
Patent: 2017562
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 6546
Estimated Expiration: ⤷ Sign Up
Patent: 3659
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PLENVU around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | E052855 | ⤷ Sign Up | |
| Eurasian Patent Organization | 037891 | СПОСОБ ОЧИЩЕНИЯ ТОЛСТОЙ КИШКИ (METHOD OF CLEANSING THE COLON) | ⤷ Sign Up |
| European Patent Office | 3116494 | MÉTHODE DE NETTOYAGE DU CÔLON (METHOD OF CLEANSING THE COLON) | ⤷ Sign Up |
| China | 103402512 | Colonoscopy - preparation | ⤷ Sign Up |
| Mexico | 2013009933 | PREPARACION PARA COLONOSCOPIA. (COLONOSCOPY - PREPARATION.) | ⤷ Sign Up |
| United Kingdom | 201307749 | ⤷ Sign Up | |
| Spain | 2928719 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PLENVU
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3141251 | C202130017 | Spain | ⤷ Sign Up | PRODUCT NAME: COMBINACION DE PRINCIPIOS ACTIVOS EN DOS DOSIS, EN LA QUE LA PRIMERA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, SULFATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO, Y LA SEGUNDA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, ACIDO ASCORBICO, ASCORBATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO.; NATIONAL AUTHORISATION NUMBER: 82959-SE/H/1801/001/DC; DATE OF AUTHORISATION: 20180525; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): IS/1/17/083/01; DATE OF FIRST AUTHORISATION IN EEA: 20171016 |
| 3141251 | 122021000018 | Germany | ⤷ Sign Up | PRODUCT NAME: EIN ARZNEIMITTEL BESTEHEND AUS EINER KOMBINATION EINER PHARMAZEUTISCHEN ZUSAMMENSETZUNG FUER EINE ERSTE DOSIS UND EINER PHARMAZEUTISCHEN ZUSAMMENSETZUNG FUER EINE ZWEITE DOSIS, WOBEI DIE PHARMAZEUTISCHE ZUSAMMENSETZUNG FUER DIE ERSTE DOSIS AUS DEN WIRKSTOFFEN POLYETHYLENGLYCOL, NATRIUMSULFAT, NATRIUMCHLORID UND KALIUMCHLORID BESTEHT UND DIE PHARMAZEUTISCHE ZUSAMMENSETZUNG FUER DIE ZWEITE DOSIS AUS DEN; NAT. REGISTRATION NO/DATE: 98450.00.00 20180205; FIRST REGISTRATION: ISLAND IS/1/17/083/01 20171016 |
| 3141251 | 301099 | Netherlands | ⤷ Sign Up | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| 3141251 | SPC/GB20/075 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS: POLYETHYLENE GLYCOL 3350, SODIUM SULPH; REGISTERED: IS IS/1/17/063/01 20171016; UK PL 20011/0040 20171016 |
| 3141251 | 132021000000044 | Italy | ⤷ Sign Up | PRODUCT NAME: UN PRODOTTO MEDICINALE COSTITUITO DA UNA COMBINAZIONE DI UNA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E UNA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA, LA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, SOLFATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO E LA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, ACIDO ASCORBICO, ASCORBATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO.(PLENVU); AUTHORISATION NUMBER(S) AND DATE(S): IS/1/17/083/01, 20171016;045671017, 045671029, 045671031, 045671043, 045671056, 20171213 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


