PIFELTRO Drug Patent Profile
✉ Email this page to a colleague
When do Pifeltro patents expire, and when can generic versions of Pifeltro launch?
Pifeltro is a drug marketed by Msd Merck Co and is included in one NDA. There is one patent protecting this drug.
This drug has seventy-five patent family members in forty-five countries.
The generic ingredient in PIFELTRO is doravirine. Two suppliers are listed for this compound. Additional details are available on the doravirine profile page.
DrugPatentWatch® Generic Entry Outlook for Pifeltro
Pifeltro was eligible for patent challenges on August 30, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 30, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PIFELTRO?
- What are the global sales for PIFELTRO?
- What is Average Wholesale Price for PIFELTRO?
Summary for PIFELTRO
| International Patents: | 75 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 57 |
| Clinical Trials: | 4 |
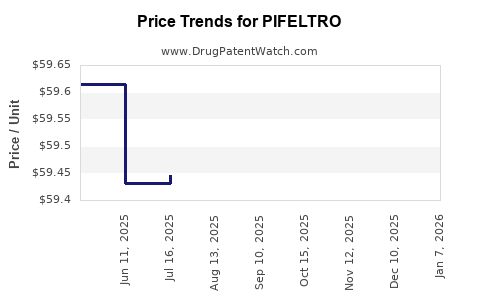
| Drug Prices: | Drug price information for PIFELTRO |
| What excipients (inactive ingredients) are in PIFELTRO? | PIFELTRO excipients list |
| DailyMed Link: | PIFELTRO at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PIFELTRO
Generic Entry Date for PIFELTRO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PIFELTRO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Chelsea and Westminster NHS Foundation Trust | Phase 3 |
| Fundación FLS de Lucha Contra el Sida, las Enfermedades Infecciosas y la Promoción de la Salud y la Ciencia | Phase 4 |
| Merck Sharp & Dohme Corp. | Phase 4 |
Pharmacology for PIFELTRO
US Patents and Regulatory Information for PIFELTRO
PIFELTRO is protected by three US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PIFELTRO is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,486,975.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd Merck Co | PIFELTRO | doravirine | TABLET;ORAL | 210806-001 | Aug 30, 2018 | RX | Yes | Yes | 8,486,975 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Msd Merck Co | PIFELTRO | doravirine | TABLET;ORAL | 210806-001 | Aug 30, 2018 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for PIFELTRO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme B.V. | Pifeltro | doravirine | EMEA/H/C/004747Pifeltro is indicated, in combination with other antiretroviral medicinal products, for the treatment of adults, and adolescents aged 12 years and older weighing at least 35 kg infected with HIV 1 without past or present evidence of resistance to the NNRTI class. | Authorised | no | no | no | 2018-11-22 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PIFELTRO
When does loss-of-exclusivity occur for PIFELTRO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0859
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11235568
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2012024691
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 94377
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12002744
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2971308
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 30126
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 120503
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150427
Estimated Expiration: ⤷ Get Started Free
Patent: 0161680
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16437
Estimated Expiration: ⤷ Get Started Free
Patent: 18774
Estimated Expiration: ⤷ Get Started Free
Patent: 19025
Estimated Expiration: ⤷ Get Started Free
Patent: 19026
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 52902
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 012000256
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 12012201
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 4804
Estimated Expiration: ⤷ Get Started Free
Patent: 1290976
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 52902
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0190020
Estimated Expiration: ⤷ Get Started Free
Patent: 0190021
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0156368
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 12002039
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 75471
Estimated Expiration: ⤷ Get Started Free
Patent: 09121
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 25336
Estimated Expiration: ⤷ Get Started Free
Patent: 31785
Estimated Expiration: ⤷ Get Started Free
Patent: 900021
Estimated Expiration: ⤷ Get Started Free
Patent: 900022
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2030
Estimated Expiration: ⤷ Get Started Free
Patent: 3334
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 81718
Estimated Expiration: ⤷ Get Started Free
Patent: 86790
Estimated Expiration: ⤷ Get Started Free
Patent: 13209405
Estimated Expiration: ⤷ Get Started Free
Patent: 13510800
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 552902
Estimated Expiration: ⤷ Get Started Free
Patent: 924034
Estimated Expiration: ⤷ Get Started Free
Patent: 2019506
Estimated Expiration: ⤷ Get Started Free
Patent: 2019507
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0113
Estimated Expiration: ⤷ Get Started Free
Patent: 0114
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3979
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 12011379
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 181
Estimated Expiration: ⤷ Get Started Free
Patent: 570
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 170
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 0980
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2670
Patent: Non-nucleoside reverse transcriptase inhibitors
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1200146
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 19018
Estimated Expiration: ⤷ Get Started Free
Patent: 19019
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 130158
Patent: INHIBIDORES NO NUCLEOSIDICOS DE LA TRANSCRIPTASA INVERSA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012501923
Patent: NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 52902
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 52902
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 017
Patent: NE-NUKLEOZIDNI INHIBITORI REVERZNE TRANSKRIPTAZE (NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Patent: 505
Patent: FARMACEUTSKA KOMPOZICIJA KOJA SADRŽI NE-NUKLEOZIDNI INHIBITOR REVERZNE TRANSKRIPTAZE (PHARMACEUTICAL COMPOSITION COMPRISING A NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 4347
Patent: NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 52902
Estimated Expiration: ⤷ Get Started Free
Patent: 24034
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1421861
Estimated Expiration: ⤷ Get Started Free
Patent: 120128703
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 36295
Estimated Expiration: ⤷ Get Started Free
Patent: 09636
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 58719
Estimated Expiration: ⤷ Get Started Free
Patent: 1139409
Patent: Non-nucleoside reverse transcriptase inhibitors
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 12000455
Patent: NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 8495
Patent: НЕНУКЛЕОЗИДНІ ІНГІБІТОРИ ЗВОРОТНОЇ ТРАНСКРИПТАЗИ (REVERSE TRANSCRIPTASE NUCLEOSIDE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PIFELTRO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Dominican Republic | P2012000256 | ⤷ Get Started Free | |
| Denmark | 2924034 | ⤷ Get Started Free | |
| Israel | 222030 | ⤷ Get Started Free | |
| Denmark | 2552902 | ⤷ Get Started Free | |
| Croatia | P20161680 | ⤷ Get Started Free | |
| Serbia | 54017 | NE-NUKLEOZIDNI INHIBITORI REVERZNE TRANSKRIPTAZE (NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS) | ⤷ Get Started Free |
| Slovenia | 2552902 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PIFELTRO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2924034 | C201930027 | Spain | ⤷ Get Started Free | PRODUCT NAME: DORAVIRINA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DE LA MISMA EN COMBINACION CON LAMIVUDINA Y TENOFOVIR DISOPROXILO FUMARATO; NATIONAL AUTHORISATION NUMBER: EU/1/18/1333; DATE OF AUTHORISATION: 20181122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1333; DATE OF FIRST AUTHORISATION IN EEA: 20181122 |
| 2552902 | 2019019 | Norway | ⤷ Get Started Free | PRODUCT NAME: DORAVIRIN ELLER ET FARMASOEYTISK AKSEPTABELT SALT DERAV; REG. NO/DATE: EU/1/18/1332/001-2 20181213 |
| 2552902 | C201930026 | Spain | ⤷ Get Started Free | PRODUCT NAME: DORAVIRINA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DE LA MISMA; NATIONAL AUTHORISATION NUMBER: EU/1/18/1333; DATE OF AUTHORISATION: 20181122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1333; DATE OF FIRST AUTHORISATION IN EEA: 20181122 |
| 2924034 | 132019000000062 | Italy | ⤷ Get Started Free | PRODUCT NAME: DORAVIRINA O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE IN COMBINAZIONE CON LAMIVUDINA O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE IN COMBINAZIONE CON TENOFOVIR O UN SUO ESTERE, IN PARTICOLARE UN ESTERE DI DISOPROXIL O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE, IN PARTICOLARE UN SALE FUMARATO(DELSTRIGO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1333/001-002, 20181127 |
| 2924034 | CR 2019 00024 | Denmark | ⤷ Get Started Free | PRODUCT NAME: DORAVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH LAMIVUDINE AND IN COMBINATION WITH TENOFOVIR DISOPROXIL FUMARATE; REG. NO/DATE: EU/1/18/1333 20181126 |
| 2924034 | LUC00114 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: DORAVIRINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI EN COMBINAISON AVEC LA LAMIVUDINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI EN COMBINAISON AVEC DU TENOFOVIR OU UN DE SES ESTERS, EN PARTICULIER UN ESTER DE DISOPROXIL OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES, EN PARTICULIER UN SEL DE FUMARATE; AUTHORISATION NUMBER AND DATE: EU/1/18/1333 20181126 |
| 2924034 | C02924034/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: DORAVIRIN, LAMIVUDIN UND TENOFOVIRDISOPROXIL; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67066 17.12.2019 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for PIFELTRO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
