PHEXXI Drug Patent Profile
✉ Email this page to a colleague
When do Phexxi patents expire, and what generic alternatives are available?
Phexxi is a drug marketed by Evofem Inc and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-five patent family members in twenty-three countries.
The generic ingredient in PHEXXI is citric acid; lactic acid; potassium bitartrate. There are five drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the citric acid; lactic acid; potassium bitartrate profile page.
DrugPatentWatch® Generic Entry Outlook for Phexxi
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for PHEXXI
| International Patents: | 65 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 3 |
| Formulation / Manufacturing: | see details |
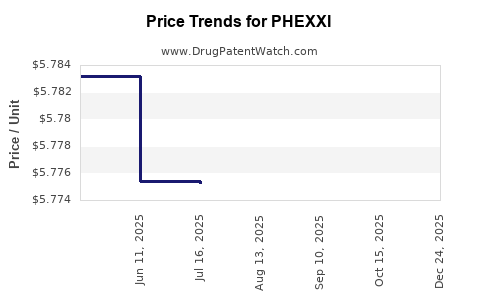
| Drug Prices: | Drug price information for PHEXXI |
| What excipients (inactive ingredients) are in PHEXXI? | PHEXXI excipients list |
| DailyMed Link: | PHEXXI at DailyMed |
Recent Clinical Trials for PHEXXI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Hawaii Foundation | Early Phase 1 |
| Queen's Medical Center | Early Phase 1 |
| Parexel | Phase 3 |
Pharmacology for PHEXXI
| Drug Class | Anti-coagulant Calculi Dissolution Agent |
| Mechanism of Action | Acidifying Activity Calcium Chelating Activity |
| Physiological Effect | Decreased Coagulation Factor Activity |
Anatomical Therapeutic Chemical (ATC) Classes for PHEXXI
Paragraph IV (Patent) Challenges for PHEXXI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PHEXXI | Vaginal Gel | citric acid; lactic acid; potassium bitartrate | 1.8%/1%/0.4% | 208352 | 1 | 2023-02-28 |
US Patents and Regulatory Information for PHEXXI
PHEXXI is protected by four US patents.
Patents protecting PHEXXI
Compositions and methods for enhancing the efficacy of contraceptive microbicides
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY
Compositions and methods for inhibiting inflammation and diseases using an alginic acid-based antimicrobial compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY
Compositions and methods for enhancing the efficacy of contraceptive microbicides
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compositions and methods for trapping and inactivating pathogenic microbes and spermatozoa
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PHEXXI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PHEXXI
See the table below for patents covering PHEXXI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2016129069 | КОМПОЗИЦИИ И СПОСОБЫ ИНГИБИРОВАНИЯ ВОСПАЛЕНИЯ И ЗАБОЛЕВАНИЙ С ПРИМЕНЕНИЕМ АНТИМИКРОБНОГО СОЕДИНЕНИЯ НА ОСНОВЕ АЛЬГИНОВОЙ КИСЛОТЫ | ⤷ Sign Up |
| Japan | 2022070924 | アルギン酸をベースとする抗菌性化合物を使用して炎症と疾患を阻害するための組成物及び方法 | ⤷ Sign Up |
| China | 106029078 | 使用基于藻酸的抗微生物化合物抑制炎症和疾病的组合物和方法 (Compositions and methods for inhibiting inflammation and diseases using an alginic acid-based antimicrobial compound) | ⤷ Sign Up |
| South Korea | 102062599 | ⤷ Sign Up | |
| European Patent Office | 3082826 | COMPOSITIONS ET MÉTHODES POUR L'INHIBITION D'UNE INFLAMMATION ET DE MALADIES À L'AIDE D'UN COMPOSÉ ANTIMICROBIEN À BASE D'ACIDE ALGINIQUE (COMPOSITIONS AND METHODS FOR INHIBITING INFLAMMATION AND DISEASES USING AN ALGINIC ACID-BASED ANTIMICROBIAL COMPOUND) | ⤷ Sign Up |
| Australia | 2001243431 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


