PARSABIV Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Parsabiv, and when can generic versions of Parsabiv launch?
Parsabiv is a drug marketed by Kai Pharms Inc and is included in one NDA. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and three patent family members in forty-four countries.
The generic ingredient in PARSABIV is etelcalcetide. One supplier is listed for this compound. Additional details are available on the etelcalcetide profile page.
DrugPatentWatch® Generic Entry Outlook for Parsabiv
Parsabiv was eligible for patent challenges on February 7, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 27, 2034. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for PARSABIV?
- What are the global sales for PARSABIV?
- What is Average Wholesale Price for PARSABIV?
Summary for PARSABIV
| International Patents: | 103 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 24 |
| Clinical Trials: | 4 |
| Patent Applications: | 39 |
| Drug Prices: | Drug price information for PARSABIV |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PARSABIV |
| What excipients (inactive ingredients) are in PARSABIV? | PARSABIV excipients list |
| DailyMed Link: | PARSABIV at DailyMed |
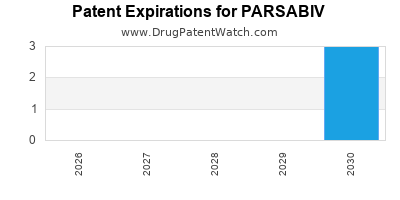

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for PARSABIV
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for PARSABIV
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Amgen | Phase 2 |
| Prim. Priv. Doz. Dr. Daniel Cejka | Phase 2 |
| Amgen | Phase 3 |
Pharmacology for PARSABIV
| Drug Class | Calcium-sensing Receptor Agonist |
| Mechanism of Action | Increased Calcium-sensing Receptor Sensitivity |
Paragraph IV (Patent) Challenges for PARSABIV
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PARSABIV | Injection | etelcalcetide | 2.5 mg/0.5 mL 5 mg/mL 10 mg/2 mL | 208325 | 2 | 2021-02-08 |
US Patents and Regulatory Information for PARSABIV
PARSABIV is protected by seven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of PARSABIV is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting PARSABIV
Stable liquid formulation of AMG 416 (etelcalcetide)
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Therapeutic agents for reducing parathyroid hormone levels
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Therapeutic agents for reducing parathyroid hormone levels
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF TREATING SECONDARY HYPERPARATHYROIDISM (SHPT)
Therapeutic agents for reducing parathyroid hormone levels
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Therapeutic agents for reducing parathyroid hormone levels
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: A METHOD OF TREATING SECONDARY HYPERPARATHYROIDISM (SHPT)
Stable liquid formulation of AMG 416 (etelcalcetide)
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kai Pharms Inc | PARSABIV | etelcalcetide | SOLUTION;INTRAVENOUS | 208325-001 | Feb 7, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Kai Pharms Inc | PARSABIV | etelcalcetide | SOLUTION;INTRAVENOUS | 208325-003 | Feb 7, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Kai Pharms Inc | PARSABIV | etelcalcetide | SOLUTION;INTRAVENOUS | 208325-001 | Feb 7, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Kai Pharms Inc | PARSABIV | etelcalcetide | SOLUTION;INTRAVENOUS | 208325-001 | Feb 7, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Kai Pharms Inc | PARSABIV | etelcalcetide | SOLUTION;INTRAVENOUS | 208325-002 | Feb 7, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for PARSABIV
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Amgen Europe B.V. | Parsabiv | etelcalcetide | EMEA/H/C/003995 Parsabiv is indicated for the treatment of secondary hyperparathyroidism (SHPT) in adult patients with chronic kidney disease (CKD) on haemodialysis therapy. |
Authorised | no | no | no | 2016-11-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PARSABIV
When does loss-of-exclusivity occur for PARSABIV?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6773
Patent: FORMULACIÓN LÍQUIDA ESTABLE
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 14302122
Patent: Stable liquid formulation of AMG 416 (Velcalcetide)
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2015032615
Patent: formulação líquida estável de amg 416 (velcalcetida)
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 16222
Patent: FORMULATION LIQUIDE STABLE D'AMG 416 (VELCALCETIDE) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 15003738
Patent: Formulación líquida estable de amg-416 (velcalcetida)
Estimated Expiration: ⤷ Sign Up
China
Patent: 5764487
Patent: AMG 416(VELCALCETIDE)的稳定的液体制剂 (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Patent: 4376970
Patent: Etelcalcetide (AMG 416)的稳定的液体制剂 (Stable liquid formulations of Etelcalcetide (AMG 416))
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 160002
Patent: FORMULACIÓN ESTABLE LÍQUIDA DE ETELCALCETIDE (AMG 461)
Estimated Expiration: ⤷ Sign Up
Patent: 160061
Patent: FORMULACIÓN ESTABLE LÍQUIDA DE ETELCALCETIDE (AMG 461)
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0171092
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 20811
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 13318
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 0220
Patent: УСТОЙЧИВАЯ ЖИДКАЯ КОМПОЗИЦИЯ, СОДЕРЖАЩАЯ AMG-416 (ЭТЕЛКАЛЦЕТИД) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Patent: 1690099
Patent: УСТОЙЧИВАЯ ЖИДКАЯ КОМПОЗИЦИЯ, СОДЕРЖАЩАЯ AMG-416 (ВЕЛКАЛЬЦЕТИД)
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 13318
Patent: FORMULATION LIQUIDE STABLE D'AMG 416 (VELCALCÉTIDE) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Patent: 46017
Patent: FORMULATION LIQUIDE STABLE D'AMG 416 HCL (ETELCALCETIDE) (STABLE LIQUID FORMULATION OF AMG 416 HCL (ETELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Patent: 78433
Patent: FORMULATION LIQUIDE STABLE D'AGONISTES PEPTIDIQUES DE SENSIBILISATEUR DE RECEPTEUR DE CALCIUM (STABLE LIQUID FORMULATION OF CALCIUM SENSING RECEPTOR PEPTIDE AGONISTS)
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 22557
Patent: 的穩定的液體製劑 (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE) AMG 416(VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 34209
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 3210
Patent: פורמולציה רוקחית יציבה של amg416(וולקלצטיד) (Stable liquid formulation of amg 416(velcalcetide))
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 27708
Estimated Expiration: ⤷ Sign Up
Patent: 16523916
Patent: 安定な液体製剤
Estimated Expiration: ⤷ Sign Up
Jordan
Patent: 17
Patent: تركيبة سائلة مستقرة ل " AMG 416 " (فيلكالسيتيد) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 13318
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 0276
Patent: STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE)
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 15017952
Patent: FORMULACION LIQUIDA ESTABLE DE AMG 416 (VELCALCETIDA). (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE).)
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 818
Patent: STAB ILNA TEČNA FORMULACIJA AMG 416 (VELKALCETIDA) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 724
Patent: Formulation liquide stable d'amg 416 (velcalcétide)
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 5403
Patent: Stable liquid formulation of amg 416 (velcalcetide)
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 160549
Patent: FORMULACION LIQUIDA ESTABLE DE ETELCALCETIDA (AMG 416)
Estimated Expiration: ⤷ Sign Up
Patent: 210413
Patent: FORMULACION LIQUIDA ESTABLE QUE COMPRENDE ETELCALCETIDA (AMG416), UN AGENTE TAMPONANTE Y AGENTE DE TONICIDAD
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 015502816
Patent: STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE)
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 13318
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 13318
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 238
Patent: STABILNA TEČNA FORMULACIJA AMG 416 (VELKALCETIDA) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201510647T
Patent: STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE)
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 13318
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1600238
Patent: STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE)
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2231957
Estimated Expiration: ⤷ Sign Up
Patent: 160043954
Patent: 에텔칼세타이드(AMG 416)의 안정한 액체 제형 (STABLE LIQUID FORMULATION OF AMG 416(VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 33989
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 35874
Estimated Expiration: ⤷ Sign Up
Patent: 1542239
Patent: Stable liquid formulation
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 15000569
Patent: STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE)
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 5373
Patent: СТАБІЛЬНИЙ РІДКИЙ СКЛАД AMG 416 (ВЕЛКАЛСЕТИДУ) (STABLE LIQUID FORMULATION OF AMG 416 (VELCALCETIDE))
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 636
Patent: FORMULACIÓN LÍQUIDA ESTABLE
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PARSABIV around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Croatia | P20190923 | ⤷ Sign Up | |
| Taiwan | 201116290 | Therapeutic agents for reducing parathyroid hormone levels | ⤷ Sign Up |
| Slovenia | 3192520 | ⤷ Sign Up | |
| Costa Rica | 20160002 | FORMULACIÓN ESTABLE LÍQUIDA DE ETELCALCETIDE (AMG 461) | ⤷ Sign Up |
| Costa Rica | 20160061 | FORMULACIÓN ESTABLE LÍQUIDA DE ETELCALCETIDE (AMG 461) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PARSABIV
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2459208 | C02459208/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: ETELCALCETID; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66338 27.09.2017 |
| 2459208 | 132017000041085 | Italy | ⤷ Sign Up | PRODUCT NAME: ETELCALCETIDE, O UN SUO SALE, COMPRESO ETELCALCETIDE IDROCLORURO(PARSABIV); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1142/001-012, 20161115 |
| 2459208 | 654 | Finland | ⤷ Sign Up | |
| 2459208 | CR 2017 00006 | Denmark | ⤷ Sign Up | PRODUCT NAME: ETELCALCETID ELLER ET SALT DERAF, INKLUSIV ETELCALCETIDHYDROCHLORID; REG. NO/DATE: EU/1/16/1142/001-012 20161115 |
| 2459208 | 1790012-7 | Sweden | ⤷ Sign Up | PRODUCT NAME: ETELCALCETIDE, OR SALT THEREOF, INCLUDING ETELCALCETIDE HYDROCHLORIDE; REG. NO/DATE: EU/1/16/1142/001-012 20161115 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


