ORKAMBI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Orkambi, and what generic alternatives are available?
Orkambi is a drug marketed by Vertex Pharms Inc and is included in two NDAs. There are twenty-one patents protecting this drug.
This drug has four hundred and fifty-six patent family members in thirty-five countries.
The generic ingredient in ORKAMBI is ivacaftor; lumacaftor. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ivacaftor; lumacaftor profile page.
DrugPatentWatch® Generic Entry Outlook for Orkambi
Orkambi was eligible for patent challenges on July 2, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 11, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for ORKAMBI?
- What are the global sales for ORKAMBI?
- What is Average Wholesale Price for ORKAMBI?
Summary for ORKAMBI
| International Patents: | 456 |
| US Patents: | 21 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 19 |
| Patent Applications: | 19 |
| Drug Prices: | Drug price information for ORKAMBI |
| What excipients (inactive ingredients) are in ORKAMBI? | ORKAMBI excipients list |
| DailyMed Link: | ORKAMBI at DailyMed |
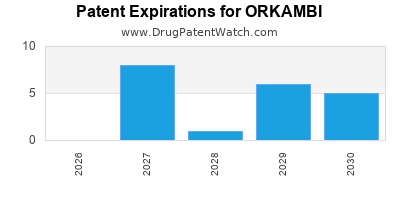

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ORKAMBI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ORKAMBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Qanatpharma Canada LTD | Phase 1 |
| Children's Hospital Medical Center, Cincinnati | N/A |
| University of North Carolina | Early Phase 1 |
Pharmacology for ORKAMBI
US Patents and Regulatory Information for ORKAMBI
ORKAMBI is protected by forty-six US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ORKAMBI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ORKAMBI
Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid and administration thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 12 YEARS OR OLDER WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE TABLET COMPRISING LUMACAFTOR AS RECITED IN CLAIM 1, 19, OR 21 OF U.S. PATENT NO. 10,076,513 AND IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING A PHARMACEUTICAL COMPOSITION ACCORDING TO CLAIM 2 OF U.S. PATENT NO. 10,597,384, FURTHER COMPRISING IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN PATIENTS 2 TO 5 YEARS OLD WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING A PHARMACEUTICAL COMPOSITION ACCORDING TO CLAIM 2 OF U.S. PATENT NO. 10,597,384, FURTHER COMPRISING IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN PATIENTS 6 YEARS AND OLDER WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING A PHARMACEUTICAL COMPOSITION ACCORDING TO CLAIM 2 OF U.S. PATENT NO. 10,597,384, FURTHER COMPRISING IVACAFTOR
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical compositions of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid and administration thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN PATIENTS 6 YEARS AND OLDER WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE TABLET ACCORDING TO CLAIM 1 OF U.S. PATENT NO. 11,052,075, WHERE THE TABLET FURTHER COMPRISES IVACAFTOR
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 TO
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 6 YEARS AND OLDER WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE COMPOSITION RECITED IN CLAIM 1 OF US 11564916
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS USING N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE AND 3-(6-(1-2,2-DIFLUOROBENZO[D][1,3]DIOXOL-5-YL) CYCLOPROPANECARBOXAMIDO)-3-METHYLPYRIDIN-2-YL)BENZOIC ACID
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING A PATIENT HAVING CYSTIC FIBROSIS USING IVACAFTOR AND LUMACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of 3-(6-(1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE DOSAGE UNIT COMPRISING LUMACAFTOR AS RECITED IN CLAIM 1 OF US PATENT 8716338 AND IVACAFTOR
Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE DOSAGE UNIT COMPRISING LUMACAFTOR AS RECITED IN CLAIM 1 OF US PATENT 8716338 AND IVACAFTOR
Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT, THE PATIENT HAVING THE F508DEL MUTATION IN CFTR, USING THE DOSAGE UNIT OF CLAIM 1 OF U.S. PATENT NO. 8,716,338
Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN PATIENTS WHO HAVE THE F508DEL MUTATION IN THE CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR (CFTR) GENE.
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND IVACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT, THE PATIENT HAVING THE F508DEL MUTATION IN CFTR, USING IVACAFTOR AND LUMACAFTOR
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN PATIENTS WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR (CFTR) GENE
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxo1-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR FORM I AND IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxo1-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR FORM I AND IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxo1-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT, THE PATIENT HAVING THE F508DEL MUTATION IN CFTR, USING IVACAFTOR AND FORM I LUMACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxo1-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN PATIENTS WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR (CFTR) GENE
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR FORM I AND IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR FORM I AND IVACAFTOR
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-- methylpyridin-2-yl) benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT, THE PATIENT HAVING THE F508DEL MUTATION IN CFTR, USING IVACAFTOR AND FORM I LUMACAFTOR
Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE DOSAGE UNIT COMPRISING LUMACAFTOR AND IVACAFTOR AS RECITED IN CLAIM 1 OF US PATENT 9192606
Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING THE DOSAGE UNIT COMPRISING LUMACAFTOR AND IVACAFTOR AS RECITED IN CLAIM 1 OF US PATENT 9192606
Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT, THE PATIENT HAVING THE F508DEL MUTATION IN CFTR, USING A DOSAGE UNIT AS DEFINED IN CLAIM 1 OF U.S. PATENT NO. 9,192,606
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND A SOLID COMPOSITION COMPRISING AMORPHOUS AND LESS THAN ABOUT 30% CRYSTALLINE IVACAFTOR
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND A SOLID COMPOSITION COMPRISING AMORPHOUS AND LESS THAN ABOUT 30% CRYSTALLINE IVACAFTOR
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING A PATIENT HAVING CYSTIC FIBROSIS USING IVACAFTOR AND LUMACAFTOR
Solid forms of N[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-- 3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 1-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND A SOLID COMPOSITION COMPRISING AMORPHOUS AND LESS THAN ABOUT 30% CRYSTALLINE IVACAFTOR
Solid forms of N[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-- 3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS IN A PATIENT AGE 2-5 YEARS WHO IS HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND A SOLID COMPOSITION COMPRISING AMORPHOUS AND LESS THAN ABOUT 30% CRYSTALLINE IVACAFTOR
Solid forms of N[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-- 3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS IN A PATIENT AGE 6 OR OLDER HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE USING LUMACAFTOR AND A SOLID COMPOSITION COMPRISING AMORPHOUS (LESS THAN ABOUT 30% CRYSTALLINE) IVACAFTOR
FDA Regulatory Exclusivity protecting ORKAMBI
NEW STRENGTH
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGED 1 YEAR TO LESS THAN 2 YEARS WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE
Exclusivity Expiration: ⤷ Sign Up
FOR THE TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGE 2 THROUGH 5 YEARS OLD WHO ARE HOMOZYGOUS FOR THE F508DEL MUTATION IN THE CFTR GENE
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | TABLET;ORAL | 206038-002 | Sep 28, 2016 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | TABLET;ORAL | 206038-001 | Jul 2, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | GRANULE;ORAL | 211358-001 | Aug 7, 2018 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | GRANULE;ORAL | 211358-001 | Aug 7, 2018 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | TABLET;ORAL | 206038-001 | Jul 2, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms Inc | ORKAMBI | ivacaftor; lumacaftor | GRANULE;ORAL | 211358-002 | Aug 7, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ORKAMBI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharmaceuticals (Ireland) Limited | Orkambi | lumacaftor, ivacaftor | EMEA/H/C/003954 Orkambi tablets are indicated for the treatment of cystic fibrosis (CF) in patients aged 6 years and older who are homozygous for the F508del mutation in the CFTR gene.Orkambi granules are indicated for the treatment of cystic fibrosis (CF) in children aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. |
Authorised | no | no | no | 2015-11-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ORKAMBI
See the table below for patents covering ORKAMBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 3219705 | ⤷ Sign Up | |
| New Zealand | 702159 | Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid | ⤷ Sign Up |
| Spain | 2619608 | ⤷ Sign Up | |
| Hong Kong | 1104292 | MODULATORS OF ATP-BINDING CASSETTE TRANSPORTERS ATP- | ⤷ Sign Up |
| New Zealand | 567892 | Heterocyclic modulators of ATP-binding cassette transporters containing cycloalkyl or heterocycloalkyl groups | ⤷ Sign Up |
| Mexico | 2012001939 | COMPOSICION FARMACEUTICA Y ADMINISTRACIONES DE LA MISMA. (PHARMACEUTICAL COMPOSITION AND ADMINISTRATIONS THEREOF.) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ORKAMBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2404919 | CA 2016 00019 | Denmark | ⤷ Sign Up | PRODUCT NAME: 3-(6-((1-(2,2-DIFLUOR-1,3-BENZODIOXOL-5-YL)CYCLOPROPANCATBONYL)AMINO)-3-METHYLPYRIDIN-2-YL)BENZOSYRE, ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, ELLER ET ESTERPRODRUG DERAF; REG. NO/DATE: EU/1/15/1059 20151124 |
| 3170818 | PA2020525,C3170818 | Lithuania | ⤷ Sign Up | PRODUCT NAME: (A) 3-(6-(1-(2,2-DIFLUORBENZO(D)(1,3)DIOKSOL-5-IL)CIKLOPROPANKARBOKSAMIDO)-3-METILPIRIDIN-2-IL)BENZENKARBOKSIRUGSTIES IR (B) N-(5-HIDROKSI-2,4-DITERT-BUTILFENIL)-4-OKSO-1H-CHINOLIN-3-KARBOKSAMIDO ARBA FARMACINIU POZIURIU PRIIMTINOS N-(5-HIDROKSI-2,4-DITERT-BUTIL-FENIL)-4-OKSO-1H-CHINOLIN-3-KARBOKSAMIDO DRUSKOS DERINYS; REGISTRATION NO/DATE: EU/1/15/1059 20151119 |
| 1773816 | 237 5014-2015 | Slovakia | ⤷ Sign Up | PRODUCT NAME: N-(5-HYDROXY-2,4-DITERC-BUTYL-FENYL)-4-OXO- -1H-CHINOLIN-3-KARBOXAMID (IVACAFTOR); REGISTRATION NO/DATE: EU/1/12/782/001 - EU/1/12/782/002 20120725 |
| 3170818 | 301060 | Netherlands | ⤷ Sign Up | PRODUCT NAME: EEN COMBINATIE VAN (A) 3-(6-(1-(2,2-DIFLUORBENZO(D)(1,3)DIOXOL-5-YL)CYCLOPROPAANCARBOXAMIDO)-3-METHYLPYRIDIN-2-YL)BENZOEZUUR EN (B) N-(5-HYDROXY-2,4-DITERT-BUTYL-FENYL)-4-OXO-1H-CHINOLINE-3-CARBOXAMIDE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/15/1059 20151124 |
| 1773816 | 2015/036 | Ireland | ⤷ Sign Up | PRODUCT NAME: N-(5-HYDROXYL-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3- CARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTRATION NO/DATE: EU/1/12/782/001-002 20120723 |
| 2404919 | PA2016015,C2404919 | Lithuania | ⤷ Sign Up | PRODUCT NAME: 3-(6-((1-(2,2-DIFLUOR-1,3-BENZODIOKSOL-5-IL)CIKLOPROPANKARBONIL)AMINO)-3-METILPIRIDIN-2-IL)BENZOINE RUGSTIS ARBA JOS FARMACINIU POZIURIU PRIIMTINA DRUSKA ARBA ESTERIS; REGISTRATION NO/DATE: EU/1/15/1059 20151119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


