ONEXTON Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Onexton, and what generic alternatives are available?
Onexton is a drug marketed by Bausch and is included in one NDA. There are five patents protecting this drug and two Paragraph IV challenges.
This drug has twenty patent family members in fourteen countries.
The generic ingredient in ONEXTON is benzoyl peroxide; clindamycin phosphate. There are seventeen drug master file entries for this compound. Fourteen suppliers are listed for this compound. Additional details are available on the benzoyl peroxide; clindamycin phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Onexton
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ONEXTON
| International Patents: | 20 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 3 |
| Clinical Trials: | 3 |
| Patent Applications: | 7 |
| Formulation / Manufacturing: | see details |
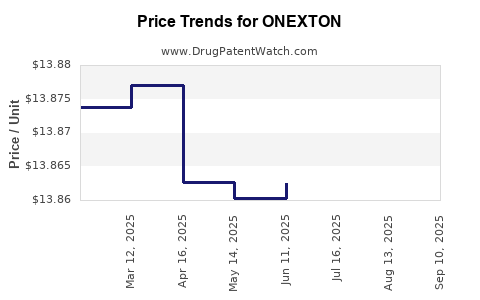
| Drug Prices: | Drug price information for ONEXTON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ONEXTON |
| What excipients (inactive ingredients) are in ONEXTON? | ONEXTON excipients list |
| DailyMed Link: | ONEXTON at DailyMed |
Recent Clinical Trials for ONEXTON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Derm Research, PLLC | Phase 4 |
| Actavis Inc. | Phase 3 |
| Taro Pharmaceuticals USA | Phase 1 |
Pharmacology for ONEXTON
| Drug Class | Lincosamide Antibacterial |
| Physiological Effect | Decreased Sebaceous Gland Activity |
Anatomical Therapeutic Chemical (ATC) Classes for ONEXTON
Paragraph IV (Patent) Challenges for ONEXTON
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ONEXTON | Gel | benzoyl peroxide; clindamycin phosphate | 1.2%/3.75% | 050819 | 1 | 2015-09-30 |
| ONEXTON | Gel | benzoyl peroxide; clindamycin phosphate | 1.2%/2.5% | 050819 | 1 | 2012-12-20 |
US Patents and Regulatory Information for ONEXTON
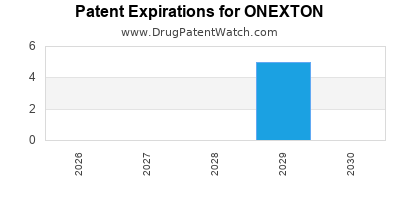
ONEXTON is protected by eleven US patents.
Patents protecting ONEXTON
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACNE VULGARIS
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACNE VULGARIS
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACNE
Topical pharmaceutical formulations containing a low concentration of benzoyl peroxide in suspension in water and a water-miscible organic solvent
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS OR OLDER
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ONEXTON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ONEXTON | benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 050819-002 | Nov 24, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ONEXTON
See the table below for patents covering ONEXTON around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Brazil | 9305884 | ⤷ Sign Up | |
| Japan | H07504168 | ⤷ Sign Up | |
| South Korea | 20110014651 | TOPICAL PHARMACEUTICAL FORMULATIONS CONTAINING A LOW CONCENTRATION OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND A WATER-MISCIBLE ORGANIC SOLVENT | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 9315726 | ⤷ Sign Up | |
| Russian Federation | 2493847 | ФАРМАЦЕВТИЧЕСКИЕ СОСТАВЫ ДЛЯ МЕСТНОГО ПРИМЕНЕНИЯ, СОДЕРЖАЩИЕ НИЗКУЮ КОНЦЕНТРАЦИЮ БЕНЗОИЛПЕРОКСИДА В СУСПЕНЗИИ В ВОДЕ И СМЕШИВАЮЩИМСЯ С ВОДОЙ ОРГАНИЧЕСКОМ РАСТВОРИТЕЛЕ (PHARMACEUTICAL FORMULATIONS FOR LOCAL APPLICATION CONTAINING LOW CONCENTRATIONS OF BENZOYL PEROXIDE IN SUSPENSION IN WATER AND WATER-MISCIBLE ORGANIC SOLVENT) | ⤷ Sign Up |
| Portugal | 626844 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ONEXTON
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1667986 | 92172 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 0591275 | SPC/GB05/030 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: NITISINONE (2-(2-NITRO-4-TRIFLUOROMETHYLBENZOYL)-1,3-CYCLOHEXANEDIONE) OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/303/001 20050221; UK EU/1/04/303/002 20050221; UK EU/1/04/303/003 20050221 |
| 1458369 | 122008000041 | Germany | ⤷ Sign Up | PRODUCT NAME: ADAPALEN IN KOMBINATION MIT BENZOYLPEROXID; NAT. REGISTRATION NO/DATE: 67913.00.00 20080229; FIRST REGISTRATION: DAENEMARK 40440 20071218 |
| 1586316 | 122011100019 | Germany | ⤷ Sign Up | PRODUCT NAME: BROMFENAC (2-AMINO-3-(4-BROMOBENZOYL)PHENYLESSIGSAEURE); REGISTRATION NO/DATE: EU/1/11/692/001 20110518 |
| 0526708 | C300097 | Netherlands | ⤷ Sign Up | PRODUCT NAME: BOSENTAN, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN HYDRAAT OF IN DE VORM VAN EEN ESTER VAN DE HYDROXYLGROEP VAN DE 2-HYDROXYETHOXY REST MET EEN ZUUR MET DE FORMULE R5-OH, WAARIN R5 EEN C1-7-ALKANOYL, BENZOYL, OF HETEROCYCLYCARBONYL VOORSTELT; NATL. REGISTRATION NO/DATE: U/1/02/220/001 - 005 20020515; FIRST REGISTRATION: CH IKS 58841 01 - 02 20020228 |
| 1458369 | SPC/GB10/005 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: ADAPALENE AND BENZOYL PEROXIDE; REGISTERED: DK 40440 20071218; UK PL10590/0057 20091111 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


