OFEV Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ofev, and when can generic versions of Ofev launch?
Ofev is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and forty-four patent family members in fifty-three countries.
The generic ingredient in OFEV is nintedanib esylate. There are nine drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the nintedanib esylate profile page.
DrugPatentWatch® Generic Entry Outlook for Ofev
Ofev was eligible for patent challenges on October 15, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 7, 2029. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (nintedanib esylate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for OFEV
| International Patents: | 244 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 75 |
| Patent Applications: | 102 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for OFEV |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OFEV |
| What excipients (inactive ingredients) are in OFEV? | OFEV excipients list |
| DailyMed Link: | OFEV at DailyMed |
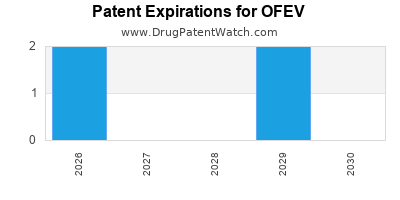

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OFEV
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for OFEV
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for OFEV
Paragraph IV (Patent) Challenges for OFEV
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OFEV | Capsules | nintedanib esylate | 100 mg and 150 mg | 205832 | 4 | 2018-10-15 |
US Patents and Regulatory Information for OFEV
OFEV is protected by five US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OFEV is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting OFEV
Pharmaceutical dosage form for immediate release of an indolinone derivative
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Medicaments for the treatment or prevention of fibrotic diseases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Substituted indolines which inhibit receptor tyrosine kinases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
3-Z-[1-(4-(N-((4-Methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-an- ilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone-monoethanesulpho- nate and the use thereof as a pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting OFEV
INDICATED TO SLOW THE RATE OF DECLINE IN PULMONARY FUNCTION IN PATIENTS WITH SYSTEMIC SCLEROSIS-ASSOCIATED INTERSTITIAL LUNG DISEASE (SSC-ILD)
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for OFEV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for OFEV
When does loss-of-exclusivity occur for OFEV?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2059
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 09254548
Estimated Expiration: ⤷ Sign Up
Patent: 15227503
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0913434
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 26267
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 10001279
Estimated Expiration: ⤷ Sign Up
China
Patent: 2056598
Estimated Expiration: ⤷ Sign Up
Patent: 5193720
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 80467
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0180709
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 20533
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 10010660
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 9996
Estimated Expiration: ⤷ Sign Up
Patent: 1001856
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 39187
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 8954
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 61031
Estimated Expiration: ⤷ Sign Up
Patent: 05542
Estimated Expiration: ⤷ Sign Up
Patent: 11522812
Estimated Expiration: ⤷ Sign Up
Patent: 14208712
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 8930
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 9229
Estimated Expiration: ⤷ Sign Up
Patent: 10013203
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 385
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 3162
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 100254
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 99987
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 142
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 99987
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1007636
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1725469
Estimated Expiration: ⤷ Sign Up
Patent: 110017872
Estimated Expiration: ⤷ Sign Up
Patent: 170020557
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 69469
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 1002691
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 10000558
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 4590
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 879
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OFEV around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Lithuania | 2878297 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 2006067165 | ⤷ Sign Up | |
| Cyprus | 1121272 | ⤷ Sign Up | |
| Eurasian Patent Organization | 015011 | ПРИМЕНЕНИЕ 3-Z-[1-(4-(N-((4-МЕТИЛПИПЕРАЗИН-1-ИЛ)МЕТИЛКАРБОНИЛ)-N-МЕТИЛАМИНО)АНИЛИНО)-1-ФЕНИЛМЕТИЛЕН]-6-МЕТОКСИКАРБОНИЛ-2-ИНДОЛИНОНА ДЛЯ ЛЕЧЕНИЯ ИЛИ ПРЕДУПРЕЖДЕНИЯ ФИБРОЗНЫХ ЗАБОЛЕВАНИЙ (USE OF 3-Z-[1-(4-(N-((4-METHYL-PIPERAZIN-1-YL)-METHYLCARBONYL)-N-METHYL-AMINO)-ANILINO)-1-PHENYL-METHYLENE]-6-METHOXYCARBONYL-2-INDOLINONE, OR THE TAUTOMERS, THE DIASTEREOMERS, THE ENANTIOMERS, THE MIXTURES THEREOF AND THE SALTS THEREOF AS MEDICAMENT FOR THE TREATMENT OR PREVENTION OF FIBROTIC DISEASES) | ⤷ Sign Up |
| Eurasian Patent Organization | 201001856 | КАПСУЛЯРНАЯ ЛЕКАРСТВЕННАЯ ФОРМА, СОДЕРЖАЩАЯ СУСПЕНЗИОННУЮ КОМПОЗИЦИЮ ПРОИЗВОДНОГО ИНДОЛИНОНА | ⤷ Sign Up |
| Canada | 2387013 | INDOLINONES SUBSTITUEES EN POSITION 6, LEUR PREPARATION ET LEUR UTILISATION EN TANT QUE COMPOSITIONS PHARMACEUTIQUES (6-POSITION SUBSTITUTED INDOLINONES, THE PREPARATION THEREOF AND THEIR USE AS PHARMACEUTICAL COMPOSITIONS) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OFEV
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1224170 | 15C0024 | France | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB,SES TAUTOMERES ET SES SELS,EN PARTICULIER LES SELS PHYSIOLOGIQUEMENT ACCEPTABLES DE NINTEDANIB ET PLUS PARTICULIEREMENT L'ESILATE; REGISTRATION NO/DATE: EU/1/14/954 20141125 |
| 1830843 | 92762 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB , OU UN TAUTOMERE, LES MELANGES OBTENUS OU UN DESES SELS, EN PARTICULIER NINTEDANIB ESILATE. FIRST REGISTRATION: 20150119 |
| 1830843 | C201530043 | Spain | ⤷ Sign Up | |
| 1224170 | SPC/GB15/018 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB*, THE TAUTOMERS AND THE SALTS THEREOF, IN PARTICULAR NINTEDANIB AND PHYSIOLOGICALLY ACCEPTABLE SALTS THEREOF, SPECIFICALLY NINTEDANIB ESILATE. *NINTEDANIB IS 3-Z-(1-(4-N-((4-METHYL-PIPERAZIN-1-YL)-METHYLCARBONYL)-N-METHYL-AMINO)-A; REGISTERED: UK EU/1/14/954/001 20141125; UK EU/1/14/954/002 20141125; UK EU/1/14/954/003 20141125; UK EU/1/14/954/004 20141125 |
| 1830843 | 15C0046 | France | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB,SES TAUTOMERES ET LEURS MELANGES AINSI QUE LES SELS PHYSIOLOGIQUEMENT ACCEPTABLES DE NINTEDANIB ET PLUS PARTICULIEREMENT L'ESILATE; REGISTRATION NO/DATE: EU/1/14/979 20150119 |
| 1224170 | CR 2015 00019 | Denmark | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB, TAUTOMERERNE OG SALTENE DERAF, SAERLIGT NINTEDANIB OG FYSIOLOGISK ACCEPTABLE SALTE DERAF, SPECIFIKT NINTEDANIB ESILAT; REG. NO/DATE: EU/1/14/954/001-004 20141121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


