OFEV Drug Patent Profile
✉ Email this page to a colleague
When do Ofev patents expire, and when can generic versions of Ofev launch?
Ofev is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and ninety-seven patent family members in fifty-three countries.
The generic ingredient in OFEV is nintedanib esylate. There are nine drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the nintedanib esylate profile page.
DrugPatentWatch® Generic Entry Outlook for Ofev
Ofev was eligible for patent challenges on October 15, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 7, 2029. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (nintedanib esylate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for OFEV?
- What are the global sales for OFEV?
- What is Average Wholesale Price for OFEV?
Summary for OFEV
| International Patents: | 197 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 76 |
| Patent Applications: | 360 |
| Drug Prices: | Drug price information for OFEV |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OFEV |
| What excipients (inactive ingredients) are in OFEV? | OFEV excipients list |
| DailyMed Link: | OFEV at DailyMed |
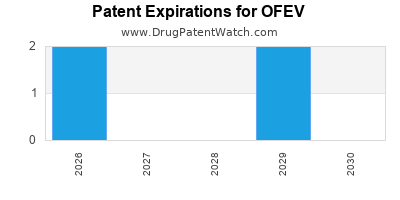

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OFEV
Generic Entry Date for OFEV*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for OFEV
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Paragraph IV (Patent) Challenges for OFEV
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OFEV | Capsules | nintedanib esylate | 100 mg and 150 mg | 205832 | 4 | 2018-10-15 |
US Patents and Regulatory Information for OFEV
OFEV is protected by four US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OFEV is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,907,756.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for OFEV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | 7,119,093 | ⤷ Get Started Free |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | 7,119,093 | ⤷ Get Started Free |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-001 | Oct 15, 2014 | 7,989,474 | ⤷ Get Started Free |
| Boehringer Ingelheim | OFEV | nintedanib esylate | CAPSULE;ORAL | 205832-002 | Oct 15, 2014 | 7,989,474 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for OFEV
When does loss-of-exclusivity occur for OFEV?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2059
Patent: FORMA DE DOSIFICACION FARMACEUTICA EN CAPSULA QUE COMPRENDE UNA FORMULACION EN SUSPENSION DE UN DERIVADO DE INDOLINONA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09254548
Patent: Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Estimated Expiration: ⤷ Get Started Free
Patent: 15227503
Patent: Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0913434
Patent: forma de dosagem farmacêutica em cápsula compreendendo uma formulação de um derivado de indolinona em supensão
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 26267
Patent: FORME POSOLOGIQUE PHARMACEUTIQUE EN CAPSULE COMPRENANT UNE FORMULATION EN SUSPENSION D'UN DERIVE D'INDOLINONE (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 10001279
Patent: Forma de dosificacion que comprende 3-z-[1-(4-(n-((4-metil-piperazin-1-il)-metilcarbonil)-n-metil-amino)-anilino)-1-fenil-metileno]-6-metoxicarbonil-2-indolinona-monoetanosulfonato, un vehiculo lipidico, un espesante y un agente de deslizamiento/solubilizante, de grupos definidos.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2056598
Patent: Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Estimated Expiration: ⤷ Get Started Free
Patent: 5193720
Patent: CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 80467
Patent: FORMA DE DOSIFICACION FARMACEUTICA EN CAPSULA QUE COMPRENDE UNA FORMULACION EN SUSPENSION DE UN DERIVADO DE INDOLINONA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180709
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20533
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010660
Patent: FORMA DE DOSIFICACIÓN FARMACÉUTICA EN CÁPSULA QUE COMPRENDE UNA FORMULACIÓN EN SUSPENCIÓN DE UN DERIVADO DE INDOLINONA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 9996
Patent: КАПСУЛЯРНАЯ ЛЕКАРСТВЕННАЯ ФОРМА, СОДЕРЖАЩАЯ СУСПЕНЗИОННУЮ КОМПОЗИЦИЮ ПРОИЗВОДНОГО ИНДОЛИНОНА (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1001856
Patent: КАПСУЛЯРНАЯ ЛЕКАРСТВЕННАЯ ФОРМА, СОДЕРЖАЩАЯ СУСПЕНЗИОННУЮ КОМПОЗИЦИЮ ПРОИЗВОДНОГО ИНДОЛИНОНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 99987
Patent: FORME POSOLOGIQUE PHARMACEUTIQUE EN CAPSULE COMPRENANT UNE FORMULATION EN SUSPENSION D'UN DÉRIVÉ D'INDOLINONE (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 39187
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8954
Patent: צורת מינון רוקחית שהינה קפסולה המכילה הרכב תרחיפי של נגזרת אינדולינון (Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 61031
Estimated Expiration: ⤷ Get Started Free
Patent: 05542
Estimated Expiration: ⤷ Get Started Free
Patent: 11522812
Estimated Expiration: ⤷ Get Started Free
Patent: 14208712
Patent: インドリノン誘導体の懸濁液製剤を含むカプセル医薬投薬形態 (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING SUSPENSION FORMULATION OF INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8930
Patent: CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9229
Patent: FORMA DE DOSIFICACIÓN FARMACÉUTICA EN CÁPSULA QUE COMPRENDE UNA FORMULACIÓN EN SUSPENSIÓN DE UN DERIVADO DE INDOLINONA. (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10013203
Patent: FORMA DE DOSIFICACION FARMACEUTICA EN CAPSULA QUE COMPRENDE UNA FORMULACION EN SUSPENSION DE UN DERIVADO DE INDOLINONA. (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 385
Patent: شكل جرعات صيدلانية لكبسولة تتألف من صياغة مستعلقة من مشتقة الإيندولينون
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3162
Patent: Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 100254
Patent: FORMA DE DOSIFICACION FARMACEUTICA EN CAPSULA QUE COMPRENDE UNA FORMULACION EN SUSPENSION DE UN DERIVADO DE INDOLINONA
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 142
Patent: FARMACEUTSKI DOZNI OBLIK U VIDU KAPSULE KOJI SADRŽI FORMULACIJU SUSPENZIJE INDOLINON DERIVATA (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 99987
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1007636
Patent: CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1725469
Estimated Expiration: ⤷ Get Started Free
Patent: 110017872
Patent: CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Patent: 170020557
Patent: 인돌리논 유도체의 현탁 제형을 포함하는 캡슐 약제학적 투여 형태 (Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 69469
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 1002691
Patent: Capsule pharmaceutical dosage form comprising a suspension formulation of an indolinone derivative
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000558
Patent: CAPSULE PHARMACEUTICAL DOAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4590
Patent: КАПСУЛИРОВАННАЯ ЛЕКАРСТВЕННАЯ ФОРМА, СОДЕРЖАЩАЯ СУСПЕНЗИОННУЮ КОМПОЗИЦИЮ ПРОИЗВОДНОЙ ИНДОЛИНОНА;КАПСУЛЬОВАНА ЛІКАРСЬКА ФОРМА, ЩО МІСТИТЬ СУСПЕНЗІЙНУ КОМПОЗИЦІЮ ПОХІДНОЇ ІНДОЛІНОНУ (CAPSULE PHARMACEUTICAL DOSAGE FORM COMPRISING A SUSPENSION FORMULATION OF AN INDOLINONE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 879
Patent: FORMA DE DOSIFICACIÓN FARMACÉUTICA EN CÁPSULA QUE COMPRENDE UNA FORMULACIÓN EN SUSPENSIÓN DE UN DERIVADO DE INDOLINONA
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OFEV around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Netherlands | 300725 | ⤷ Get Started Free | |
| Poland | 355433 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2004017948 | ⤷ Get Started Free | |
| Croatia | P20190181 | ⤷ Get Started Free | |
| European Patent Office | 2281561 | Utilisation d'inhibiteur LCK pour le traitement d'affectations immunologiques (Use of LCK inhibitors for treatment of immunologic diseases) | ⤷ Get Started Free |
| Ukraine | 107560 | ФАРМАЦЕВТИЧНА ЛІКАРСЬКА ФОРМА ДЛЯ НЕГАЙНОГО ВИВІЛЬНЕННЯ ПОХІДНОЇ ІНДОЛІНОНУ | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OFEV
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1830843 | C20150026 00160 | Estonia | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIIB;REG NO/DATE: EU/1/14/979 19.01.2015 |
| 1830843 | 300747 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIB, OF EEN TAUTOMEER, DE MENGSELS DAARVAN OF EEN ZOUT DAARVAN; IN HET BIJZONDER NINTEDANIBESILAAT; REGISTRATION NO/DATE: EU/1/14/979 20150115 |
| 1830843 | 92762 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIB , OU UN TAUTOMERE, LES MELANGES OBTENUS OU UN DESES SELS, EN PARTICULIER NINTEDANIB ESILATE. FIRST REGISTRATION: 20150119 |
| 1830843 | PA2015025 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIBUM; REGISTRATION NO/DATE: EU/1/14/979/001 - EU/1/14/979/004 20150115 |
| 1830843 | C 2015 026 | Romania | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIB SAU UN TAUTOMER SAU AMESTECURI ALE ACESTORA SAU O SARE A ACESTORA, IN SPECIAL ESILAT DE NINTEDANIB; NATIONAL AUTHORISATION NUMBER: EU/1/14/979/001-004; DATE OF NATIONAL AUTHORISATION: 20150115; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/979/001-004; DATE OF FIRST AUTHORISATION IN EEA: 20150115 |
| 1224170 | 15C0024 | France | ⤷ Get Started Free | PRODUCT NAME: NINTEDANIB,SES TAUTOMERES ET SES SELS,EN PARTICULIER LES SELS PHYSIOLOGIQUEMENT ACCEPTABLES DE NINTEDANIB ET PLUS PARTICULIEREMENT L'ESILATE; REGISTRATION NO/DATE: EU/1/14/954 20141125 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for OFEV (Nintedanib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
