NUEDEXTA Drug Patent Profile
✉ Email this page to a colleague
When do Nuedexta patents expire, and when can generic versions of Nuedexta launch?
Nuedexta is a drug marketed by Avanir Pharms and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has seventy-five patent family members in twenty-one countries.
The generic ingredient in NUEDEXTA is dextromethorphan hydrobromide; quinidine sulfate. There are twenty-three drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the dextromethorphan hydrobromide; quinidine sulfate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nuedexta
A generic version of NUEDEXTA was approved as dextromethorphan hydrobromide; quinidine sulfate by ACTAVIS ELIZABETH on October 10th, 2017.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for NUEDEXTA?
- What are the global sales for NUEDEXTA?
- What is Average Wholesale Price for NUEDEXTA?
Summary for NUEDEXTA
| International Patents: | 75 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 11 |
| Patent Applications: | 149 |
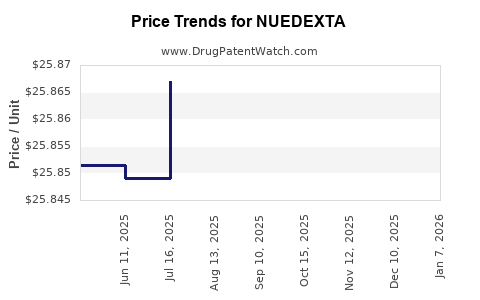
| Drug Prices: | Drug price information for NUEDEXTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NUEDEXTA |
| What excipients (inactive ingredients) are in NUEDEXTA? | NUEDEXTA excipients list |
| DailyMed Link: | NUEDEXTA at DailyMed |
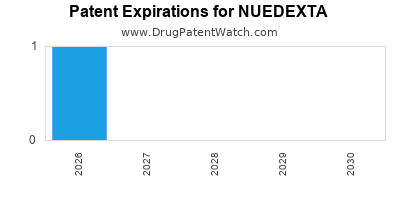
Paragraph IV (Patent) Challenges for NUEDEXTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NUEDEXTA | Capsules | dextromethorphan hydrobromide; quinidine sulfate | 20 mg/10 mg | 021879 | 1 | 2011-03-07 |
US Patents and Regulatory Information for NUEDEXTA
NUEDEXTA is protected by one US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avanir Pharms | NUEDEXTA | dextromethorphan hydrobromide; quinidine sulfate | CAPSULE;ORAL | 021879-001 | Oct 29, 2010 | AB | RX | Yes | Yes | 7,659,282 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NUEDEXTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Avanir Pharms | NUEDEXTA | dextromethorphan hydrobromide; quinidine sulfate | CAPSULE;ORAL | 021879-001 | Oct 29, 2010 | 5,166,207 | ⤷ Get Started Free |
| Avanir Pharms | NUEDEXTA | dextromethorphan hydrobromide; quinidine sulfate | CAPSULE;ORAL | 021879-001 | Oct 29, 2010 | RE38115 | ⤷ Get Started Free |
| Avanir Pharms | NUEDEXTA | dextromethorphan hydrobromide; quinidine sulfate | CAPSULE;ORAL | 021879-001 | Oct 29, 2010 | 5,206,248 | ⤷ Get Started Free |
| Avanir Pharms | NUEDEXTA | dextromethorphan hydrobromide; quinidine sulfate | CAPSULE;ORAL | 021879-001 | Oct 29, 2010 | 8,227,484 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for NUEDEXTA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Jenson Pharmaceutical Services Limited | Nuedexta | dextromethorphan hydrobromide, quinidine sulfate | EMEA/H/C/002560Nuedexta is indicated for the symptomatic treatment of pseudobulbar affect (PBA) in adults. Efficacy has only been studied in patients with underlying amyotrophic lateral sclerosis or multiple sclerosis. | Withdrawn | no | no | no | 2013-06-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for NUEDEXTA
See the table below for patents covering NUEDEXTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2017202884 | Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of neurological disorders | ⤷ Get Started Free |
| Hungary | E028100 | ⤷ Get Started Free | |
| Russian Federation | 2341265 | ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ ДЛЯ ЛЕЧЕНИЯ НЕВРОЛОГИЧЕСКИХ РАССТРОЙСТВ, СОДЕРЖАЩИЕ ДЕКСТРОМЕТОРФАН И ХИНИДИН (PHARMACEUTICAL COMPOSITIONS FOR NEURAL DISORDER TREATMENT, CONTAINING DEXTROMETHORPHANE AND QUINIDINE) | ⤷ Get Started Free |
| Australia | 2015203262 | Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of neurological disorders D2 | ⤷ Get Started Free |
| European Patent Office | 3824889 | COMPOSITIONS PHARMACEUTIQUES COMPRENANT DU DEXTROMÉTHORPHAN ET DE LA QUINIDINE POUR LE TRAITEMENT DE L'INSTABILITÉ ÉMOTIONNELLE (PHARMACEUTICAL COMPOSITIONS COMPRISING DEXTROMETHORPHAN AND QUINIDINE FOR THE TREATMENT OF EMOTIONAL LABILITY) | ⤷ Get Started Free |
| Australia | 2013202187 | Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of neurological disorders - D1 | ⤷ Get Started Free |
| South Korea | 20190143466 | 덱스트로메토르판 및 퀴니딘을 포함하는 신경계 장애의 치료를 위한 약학적 조성물 (Pharmaceutical composition comprising dextromethorphan and quinidine for the treatment of neurological disorders) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NUEDEXTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1539166 | 2013/055 | Ireland | ⤷ Get Started Free | PRODUCT NAME: THE COMBINATION OF: (A) DEXTROMETHORPHAN OR A PHARMACEUTICALLY ACCEPTABLE SALT, PRECURSOR OR DERIVATIVE THEREOF, E.G. DEXTROMETHORPHAN HYDROBROMIDE AND IN PARTICULAR DEXTROMETHORPHAN HYDROBROMIDE MONOHYDRATE; AND (B) QUINIDINE OR A PHARMACEUTICALLY ACCEPTABLE SALT, PRECURSOR OR DERIVATIVE THEREOF, E.G. QUINIDINE SULPHATE AND IN PARTICULAR QUINIDINE SULPHATE DIHYDRATE, PROTECTED BY THE BASIC PATENT; REGISTRATION NO/DATE: EU/1/13/833 20130624 |
| 1539166 | 60/2013 | Austria | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION VON A) DEXTROMETHORPHAN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, BEISPIELSWEISE DEXTROMETHORPHANHYDROBROMID, UND INSBESONDERE DEXTROMETHORPHANHYDROBROMIDMONOHYDRAT UND B) CHINIDIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON, BEISPIELSWEISE CHINIDINSULFAT, UND INSBESONDERE CHINIDINSULFATDIHYDRAT.; REGISTRATION NO/DATE: EU/1/13/833 20130626 |
| 1539166 | C20130030 00105 | Estonia | ⤷ Get Started Free | PRODUCT NAME: DEKSTROMETORFAAN / KINIDIIN;REG NO/DATE: K(2013)4096 (LOPLIK) 26.06.2013 |
| 1539166 | 122013000090 | Germany | ⤷ Get Started Free | PRODUCT NAME: KOMBINATION AUS: (A) DEXTROMETHORPHAN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ, Z.B. DEXTROMETHORPHAN-HYDROBROMID UND INSBESONDERE DEXTROMETHORPHAN-HYDROBROMID-MONOHYDRAT; UND (B) CHINIDIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, Z.B.CHINIDIN-SULFAT UND INSBESONDERE CHINIDIN-SULFAT-DIHYDRAT; REGISTRATION NO/DATE: EU/1/13/833 20130624 |
| 1539166 | C300626 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE VAN: (A) DEXTROMETHORFAN, DESGEWENST; REGISTRATION NO/DATE: EU/1/13/833 20130624 |
| 1539166 | C 2013 034 | Romania | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE A DEXTROMETORFANULUI SAU A UNEI SARIACCEPTABILE FARMACEUTIC, DE EXEMPLU DEXNATIONAL AUTHORISATION NUMBER: EU/1/13/833/001, EU/1/13/833/002, EU/1/13/833/003; DATE OF NATIONAL AUTHORISATION: 20130624; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/833/001, EU/1/13/833/002, EU/1/13/833/003; DATE OF FIRST AUTHORISATION IN EEA: 20130624 TROMETORFAN BROMHIDRAT SI PARTICULAR DEXTROMETORFAN BROMHIDRATMONOHIDRAT SI CHINIDINA SAU O SARE ACCEPTABILA FARMACEUTIC, DE EXEMPLU SULFAT DE CHINIDINA SI IN PAR TICULAR SULFATDE CHINIDINA DIHIDRAT; |
| 1539166 | 132013902215214 | Italy | ⤷ Get Started Free | PRODUCT NAME: ASSOCIAZIONE DI (A) DESTROMETORFANO O UN SUO SALE, PRECURSORE O DERIVATO FARMACEUTICAMENTE ACCETTABILE, INCLUSO IL DESTROMETORFANO BROMIDRATO E IN PARTICOLARE IL DESTROMETORFANO BROMIDRATO MONOIDRATO E (B) CHINIDINA O UN SUO SALE,PRECURSORE O DERIVATO FARMACEUTICAMENTE ACCETTABILE, INCLUSA LA CHINIDINA SOLFATO E IN PARTICOLARE LA CHINIDINA SOLFATO DIIDRATO(NUEDEXTA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/833, 20130626 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Nuedexta (Dextromethorphan, Quinine)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
