NOURIANZ Drug Patent Profile
✉ Email this page to a colleague
When do Nourianz patents expire, and what generic alternatives are available?
Nourianz is a drug marketed by Kyowa Kirin and is included in one NDA. There are three patents protecting this drug.
This drug has sixty-three patent family members in eighteen countries.
The generic ingredient in NOURIANZ is istradefylline. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the istradefylline profile page.
DrugPatentWatch® Generic Entry Outlook for Nourianz
Nourianz was eligible for patent challenges on August 27, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 13, 2024. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for NOURIANZ
| International Patents: | 63 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 92 |
| Clinical Trials: | 3 |
| Patent Applications: | 448 |
| Drug Prices: | Drug price information for NOURIANZ |
| What excipients (inactive ingredients) are in NOURIANZ? | NOURIANZ excipients list |
| DailyMed Link: | NOURIANZ at DailyMed |
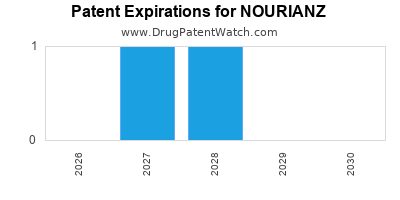

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for NOURIANZ
Generic Entry Date for NOURIANZ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for NOURIANZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Kyowa Kirin, Inc. | Phase 4 |
| Georgetown University | Phase 4 |
| ALS Association | Phase 1/Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for NOURIANZ
US Patents and Regulatory Information for NOURIANZ
NOURIANZ is protected by three US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of NOURIANZ is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting NOURIANZ
Microcrystal
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Administering adenosine A.sub.2A receptor antagonist to reduce or suppress side effects of parkinson's disease therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF REDUCING OFF TIME FROM L-DOPA THERAPY, COMPRISING ADMINISTERING, TO A HUMAN PATIENT WITH PARKINSON'S DISEASE, AN EFFECTIVE AMOUNT OF ISTRADEFYLLINE, WHEREIN THE PATIENT CURRENTLY RECEIVES SAID L-DOPA THERAPY
Method of stabilizing diarylvinylene compound
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting NOURIANZ
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-001 | Aug 27, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-002 | Aug 27, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-001 | Aug 27, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-002 | Aug 27, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-001 | Aug 27, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NOURIANZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-001 | Aug 27, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Kyowa Kirin | NOURIANZ | istradefylline | TABLET;ORAL | 022075-002 | Aug 27, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for NOURIANZ
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Kyowa Kirin Holdings B.V. | Nouryant | istradefylline | EMEA/H/C/005308 Istradefylline is indicated in adults as an adjunctive treatment to levodopa based regimens in patients with Parkinson’s disease (PD) experiencing “OFF” time. |
Refused | no | no | no | 2022-01-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for NOURIANZ
See the table below for patents covering NOURIANZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1469855 | ⤷ Try a Trial | |
| Japan | 4376630 | ⤷ Try a Trial | |
| Japan | 4606326 | ⤷ Try a Trial | |
| China | 101822676 | ⤷ Try a Trial | |
| Canada | 2473864 | COMPOSITION DE TRAITEMENT DES PATIENTS SOUFFRANT DE TROUBLES MOTEURS (COMPOSITION FOR USE IN TREATING PATIENTS SUFFERING FROM MOVEMENT DISORDER) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


