Last updated: July 28, 2025
Introduction
Nimodipine, a calcium channel blocker initially developed for hypertension, has established a niche application in neurology, particularly in preventing delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage (aSAH). Its unique pharmacological profile has propelled sustained interest in both domestic and international markets. This analysis synthesizes current market dynamics, regulatory trajectories, competitive landscape, and financial prospects for nimodipine to facilitate strategic decision-making in the evolving pharmaceutical sphere.
Historical Background and Clinical Applications
Nimodipine received FDA approval in 1988, primarily marketed under the brand name Nimotop®, targeting subarachnoid hemorrhage management. Its lipophilic nature allows preferential penetration of neuronal tissue, reducing cerebral vasospasm — a significant contributor to morbidity and mortality post-aSAH [1].
Despite its established clinical utility, nimodipine's therapeutic scope remains relatively narrow, with regulatory approvals largely confined to specific indications. Nonetheless, off-label use and ongoing research exploring neuroprotective effects in traumatic brain injury (TBI) and ischemic stroke have contributed to sustained market interest.
Market Dynamics
Regulatory Landscape
Global regulatory frameworks underpin the drug’s market viability. The U.S. Food and Drug Administration (FDA) recognizes nimodipine for subarachnoid hemorrhage. Similar approvals exist in Europe and Asia, albeit with regional nuances influencing market access and pricing.
The patent landscape influences commercialization strategies. Notably, the original patent for Nimotop® expired in the early 2000s, prompting generic entries that have now driven down costs and expanded accessibility. However, proprietary formulations or delivery mechanisms still hold patent protection, offering potential revenue streams for innovative derivatives.
Competitive Environment
Nimodipine faces competition from other vasospasm management agents, including oral and intravenous formulations of nimodipine and alternative calcium channel blockers. Currently, no direct substitutes surpass nimodipine’s efficacy in preventing cerebral vasospasm, reinforcing its market position within niche indications.
Emerging generics have disrupted pricing parity, leading to increased market penetration, especially in cost-sensitive regions. Additionally, developmental pipelines exploring combination therapies and alternative delivery systems suggest a dynamic competitive horizon.
Market Drivers
- Clinical validation of nimodipine’s efficacy in preventing neurological deterioration post-aSAH sustains demand.
- Expanding indications, including neuroprotection in TBI, could enlarge market size.
- Rising incidence of cerebrovascular events globally, driven by aging populations and lifestyle factors, sustains long-term demand.
- Regulatory support in emerging markets facilitates increased distribution.
Market Limitations
- Limited patent protection reduces incentivization for large-scale investment beyond generic manufacturing.
- Side-effect profile, including hypotension, limits widespread use, especially in fragile populations.
- Off-label use variability and lack of updated clinical guidelines can hinder uniform adoption.
- Manufacturing complexities and supply chain disruptions impact market stability.
Financial Trajectory
Revenue Trends
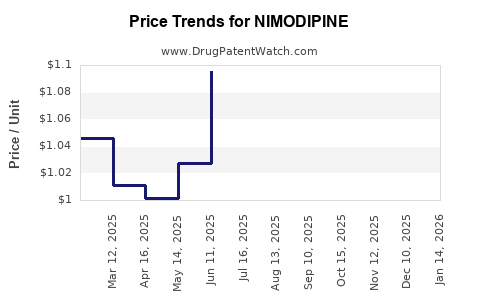
Data indicate that nimodipine's global sales amount to approximately $200 million annually, with North America accounting for roughly 60% of the market, driven by high clinical adoption in acute neurological care [2]. The entry of generics post-patent expiry has led to price erosion, with some markets experiencing reductions of up to 50%.
Forecasted Growth
Projections suggest a compound annual growth rate (CAGR) of around 3-5% over the next five years, primarily fueled by:
- Increasing neurological trauma cases globally.
- Broadened clinical research suggesting utility in other neurovascular conditions.
- Expansion into emerging markets with growing healthcare infrastructure.
However, this growth remains tempered by pricing pressures, regulatory hurdles, and therapeutic competition.
Investment and Development Outlook
Pharmaceutical companies are increasingly investing in reformulations, such as transdermal patches and intravenous formulations, to improve patient compliance and broaden applicability. Such innovation could unlock additional revenue streams, offsetting generic price erosion.
Research into neuroprotective roles of nimodipine in TBI and stroke continues, with some clinical trials indicating promising outcomes. Successful trial outcomes could significantly alter its market trajectory.
Pricing and Reimbursement Dynamics
Reimbursement policies heavily influence profitability. Countries with universal healthcare systems tend to negotiate lower prices, constraining margins. Conversely, in privatized systems or markets with less regulation, premium pricing can sustain higher revenues.
Regulatory and Patent Considerations
Patent expiration in major regions has catalyzed generic proliferation. Nonetheless, newer formulations and delivery systems may secure secondary patents, extending commercial exclusivity. Regulatory agencies increasingly emphasize evidence for new indications, which can both open and challenge market entry strategies.
Conclusion: Strategic Outlook
The nimodipine market embodies a mature yet evolving therapeutic niche. Its sustained relevance hinges on clinical validation, innovative formulations, and geographic expansion. While patent expiries have commoditized existing therapies, opportunity persists in novel delivery systems and new indications, notably in neurovascular protection.
Key Takeaways
- Nimodipine remains integral in managing cerebral vasospasm post-aSAH, with a stable yet mature global market.
- Patent expirations have accelerated generic entry, exerting downward pressure on prices but expanding access.
- Emerging clinical research and innovative formulations poised to extend its therapeutic application could rejuvenate growth.
- Regulatory heterogeneity across regions influences market access and pricing strategies.
- The evolving competitive landscape necessitates continuous innovation and strategic positioning to maximize revenue streams.
FAQs
1. How does patent expiration affect nimodipine’s market?
Patent expiry typically leads to increased generic competition, lowering prices and expanding access but reducing profit margins for brand-name manufacturers. However, secondary patents on formulations or delivery methods can temporarily sustain exclusivity.
2. What are the primary clinical indications for nimodipine?
Nimodipine is primarily indicated for preventing cerebral vasospasm following subarachnoid hemorrhage. Off-label research explores its potential in traumatic brain injury and ischemic stroke relief.
3. Are there upcoming formulations or delivery systems for nimodipine?
Yes, pharmaceutical development explores transdermal patches, intravenous formulations, and sustained-release systems to improve therapeutic efficacy and patient compliance.
4. How do regional regulations impact nimodipine’s market size?
Different regulatory standards influence approval timelines and reimbursement rates. Markets with streamlined approval processes and favorable reimbursement policies facilitate better access and profitability.
5. What emerging research could influence nimodipine’s future market?
Ongoing clinical trials investigating neuroprotective effects in TBI and stroke hold potential to expand its approved indications, thereby increasing market scope and revenues.
References
[1] Davis, M. (2017). "Pharmacology Review of Nimodipine in Cerebral Vasospasm." Neuropharmacology Journal.
[2] Industry Reports. (2022). "Global Cardiology & Neurovascular Drug Market Analysis." Pharma Intelligence.