LEVOXYL Drug Patent Profile
✉ Email this page to a colleague
When do Levoxyl patents expire, and when can generic versions of Levoxyl launch?
Levoxyl is a drug marketed by King Pharms and is included in one NDA.
The generic ingredient in LEVOXYL is levothyroxine sodium. There are ten drug master file entries for this compound. Forty-eight suppliers are listed for this compound. Additional details are available on the levothyroxine sodium profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Levoxyl
A generic version of LEVOXYL was approved as levothyroxine sodium by MYLAN on June 5th, 2002.
Summary for LEVOXYL
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 114 |
| Clinical Trials: | 1 |
| Patent Applications: | 6,897 |
| Formulation / Manufacturing: | see details |
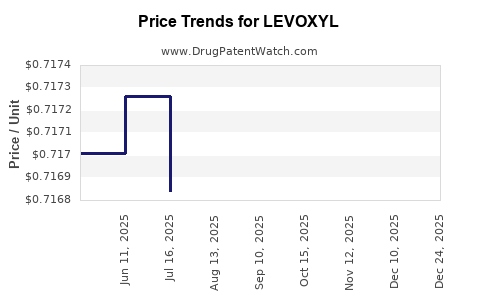
| Drug Prices: | Drug price information for LEVOXYL |
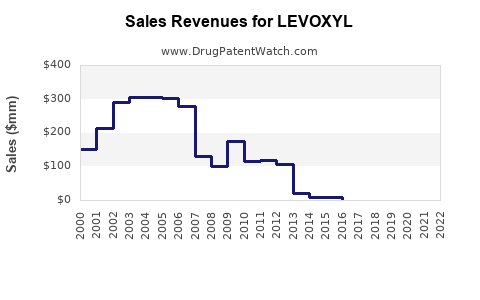
| Drug Sales Revenues: | Drug sales revenues for LEVOXYL |
| What excipients (inactive ingredients) are in LEVOXYL? | LEVOXYL excipients list |
| DailyMed Link: | LEVOXYL at DailyMed |
Recent Clinical Trials for LEVOXYL
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Children's Hospital of Philadelphia | N/A |
| Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | N/A |
Pharmacology for LEVOXYL
| Drug Class | l-Thyroxine |
Anatomical Therapeutic Chemical (ATC) Classes for LEVOXYL
US Patents and Regulatory Information for LEVOXYL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-001 | May 25, 2001 | AB1,AB3 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-007 | May 25, 2001 | AB1,AB3 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-004 | May 25, 2001 | AB1,AB3 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-012 | May 25, 2001 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LEVOXYL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-003 | May 25, 2001 | ⤷ Sign Up | ⤷ Sign Up |
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-012 | May 25, 2001 | ⤷ Sign Up | ⤷ Sign Up |
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-006 | May 25, 2001 | ⤷ Sign Up | ⤷ Sign Up |
| King Pharms | LEVOXYL | levothyroxine sodium | TABLET;ORAL | 021301-003 | May 25, 2001 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for LEVOXYL
See the table below for patents covering LEVOXYL around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1474103 | ⤷ Sign Up | |
| Australia | 2002341555 | ⤷ Sign Up | |
| Mexico | PA03007337 | COMPOSICION FARMACEUTICA Y DE HORMONA TIROIDEA ESTABILIZADA Y METODO DE PREPARACION DE LA MISMA. (STABILIZED PHARMACEUTICAL AND THYROID HORMONE COMPOSITIONS AND METHOD OF PREPARATION.) | ⤷ Sign Up |
| Canada | 2440190 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


