KISQALI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Kisqali, and what generic alternatives are available?
Kisqali is a drug marketed by Novartis and is included in two NDAs. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eighty-four patent family members in fifty-three countries.
The generic ingredient in KISQALI is letrozole; ribociclib succinate. There are twenty-four drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the letrozole; ribociclib succinate profile page.
DrugPatentWatch® Generic Entry Outlook for Kisqali
Kisqali was eligible for patent challenges on March 13, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 14, 2036. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for KISQALI
| International Patents: | 184 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 140 |
| Clinical Trials: | 33 |
| Patent Applications: | 1,159 |
| Drug Prices: | Drug price information for KISQALI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KISQALI |
| What excipients (inactive ingredients) are in KISQALI? | KISQALI excipients list |
| DailyMed Link: | KISQALI at DailyMed |
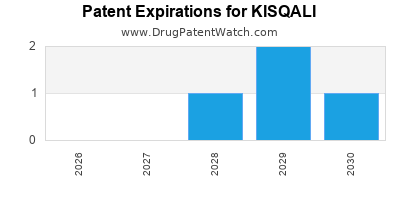

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KISQALI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for KISQALI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Nationwide Children's Hospital | Phase 2 |
| Karolinska University Hospital | Phase 2 |
| Relay Therapeutics, Inc. | Phase 1 |
Pharmacology for KISQALI
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 3A Inhibitors Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for KISQALI
Paragraph IV (Patent) Challenges for KISQALI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KISQALI | Tablets | ribociclib succinate | 200 mg | 209092 | 4 | 2021-03-15 |
US Patents and Regulatory Information for KISQALI
KISQALI is protected by twenty US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KISQALI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting KISQALI
Ribociclib tablet
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR TREATMENT OF POSTMENOPAUSAL WOMEN WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER-2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT FOR THE TREATMENT OF POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR FOR THE TREATMENT OF PRE/PERIMENOPAUSAL OR POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE-BASED THERAPY
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR THE TREATMENT OF ADULT PATIENTS WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT AS INITIAL ENDOCRINE-BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY IN POSTMENOPAUSAL WOMEN OR IN MEN, FOR THE TREATMENT OF HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-D]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR FOR THE TREATMENT OF PRE/PERIMENOPAUSAL OR POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE-BASED THERAPY
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT FOR THE TREATMENT OF POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR TREATMENT OF POSTMENOPAUSAL WOMEN WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER-2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT AS INITIAL ENDOCRINE-BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY IN POSTMENOPAUSAL WOMEN OR IN MEN, FOR THE TREATMENT OF HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Pyrrolopyrimidine compounds and their uses
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR THE TREATMENT OF ADULT PATIENTS WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR FOR THE TREATMENT OF PRE/PERIMENOPAUSAL OR POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE-BASED THERAPY
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT FOR THE TREATMENT OF POSTMENOPAUSAL WOMEN WITH HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER, AS INITIAL ENDOCRINE BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR TREATMENT OF POSTMENOPAUSAL WOMEN WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER-2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH AN AROMATASE INHIBITOR AS INITIAL ENDOCRINE-BASED THERAPY FOR THE TREATMENT OF ADULT PATIENTS WITH HORMONE RECEPTOR (HR)-POSITIVE, HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 (HER2)-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
Salt(s) of 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo- [2,3-d]pyrimidine-6-carboxylic acid dimethylamide and processes of making thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: IN COMBINATION WITH FULVESTRANT AS INITIAL ENDOCRINE-BASED THERAPY OR FOLLOWING DISEASE PROGRESSION ON ENDOCRINE THERAPY IN POSTMENOPAUSAL WOMEN OR IN MEN, FOR THE TREATMENT OF HR-POSITIVE, HER2-NEGATIVE ADVANCED OR METASTATIC BREAST CANCER
FDA Regulatory Exclusivity protecting KISQALI
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | KISQALI FEMARA CO-PACK (COPACKAGED) | letrozole; ribociclib succinate | TABLET;ORAL | 209935-001 | May 4, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | KISQALI FEMARA CO-PACK (COPACKAGED) | letrozole; ribociclib succinate | TABLET;ORAL | 209935-001 | May 4, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novartis | KISQALI FEMARA CO-PACK (COPACKAGED) | letrozole; ribociclib succinate | TABLET;ORAL | 209935-001 | May 4, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Novartis | KISQALI FEMARA CO-PACK (COPACKAGED) | letrozole; ribociclib succinate | TABLET;ORAL | 209935-001 | May 4, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novartis | KISQALI | ribociclib succinate | TABLET;ORAL | 209092-001 | Mar 13, 2017 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for KISQALI
When does loss-of-exclusivity occur for KISQALI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4257
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 16248017
Estimated Expiration: ⤷ Sign Up
Patent: 19201929
Estimated Expiration: ⤷ Sign Up
Patent: 20250190
Estimated Expiration: ⤷ Sign Up
Patent: 22215155
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2017021283
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 82425
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 17002593
Estimated Expiration: ⤷ Sign Up
China
Patent: 7530292
Estimated Expiration: ⤷ Sign Up
Patent: 5554257
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 17010510
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0230053
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 83058
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 17075052
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1792290
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 83058
Estimated Expiration: ⤷ Sign Up
Patent: 97530
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 83058
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 61213
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 18514523
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 17013350
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 180035
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 017501820
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 83058
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 83058
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201708084P
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 83058
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 170137101
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 38261
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 1642864
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 17000422
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KISQALI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Montenegro | 01282 | JEDINJENJA PIROLOPIRIMIDINA KAO INHIBITORI CDK (PYRROLOPYRIMIDINE COMPOUNDS AS CDK INHIBITORS) | ⤷ Sign Up |
| Croatia | P20170631 | ⤷ Sign Up | |
| Argentina | 073116 | COMPUESTOS DE PIRROLO[2,3-D]-PIRIMIDINA MODULADORES DE PROTEINQUINASAS CDK, COMPOSICIONES FARMACEUTICAS QUE LOS CONTIENEN Y USO DE LOS MISMOS EN EL TRATAMIENTO Y/O PREVENCION DE CANCER Y ENFERMEDADES AUTOINMUNES. | ⤷ Sign Up |
| Tunisia | 2011000062 | PYRROLOPYRIMIDINE COMPOUNDS OF CDK INHIBITORS | ⤷ Sign Up |
| Chile | 2011000306 | Compuestos derivados de di-alquilamida del acido 7-ciclopentil-2-[5-(piperidin o piperazin sustituido)-piridin-2-il amino]-7h-pirrolo-[2,3-d]-pirimidin-6-carboxilico, inhibidores de cdk4; composicion farmaceutica; y su uso para el tratamiento del cancer o la inflamacion. | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KISQALI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2331547 | 17C1059 | France | ⤷ Sign Up | PRODUCT NAME: RIBOCICLIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; REGISTRATION NO/DATE: EU/1/17/1221 20170824 |
| 2331547 | PA2017039,C2331547 | Lithuania | ⤷ Sign Up | PRODUCT NAME: RIBOCIKLIBAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/17/1221 20170822 |
| 2331547 | LUC00048 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: RIBOCICLIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/171221 20170824 |
| 2331547 | 2017/060 | Ireland | ⤷ Sign Up | PRODUCT NAME: RIBOCICLIB OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTRATION NO/DATE: EU/1/17/1221 20170824 |
| 2331547 | SPC/GB17/074 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: RIBOCICLIB OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/17/1221/001-012 20170824; UK PLGB 00101/1100 20170824 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


