INQOVI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Inqovi, and when can generic versions of Inqovi launch?
Inqovi is a drug marketed by Taiho Oncology and is included in one NDA. There are six patents protecting this drug.
This drug has eighty-nine patent family members in forty-one countries.
The generic ingredient in INQOVI is cedazuridine; decitabine. One supplier is listed for this compound. Additional details are available on the cedazuridine; decitabine profile page.
DrugPatentWatch® Generic Entry Outlook for Inqovi
Inqovi was eligible for patent challenges on July 7, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 22, 2030. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for INQOVI?
- What are the global sales for INQOVI?
- What is Average Wholesale Price for INQOVI?
Summary for INQOVI
| International Patents: | 89 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 15 |
| Clinical Trials: | 28 |
| Drug Prices: | Drug price information for INQOVI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for INQOVI |
| What excipients (inactive ingredients) are in INQOVI? | INQOVI excipients list |
| DailyMed Link: | INQOVI at DailyMed |
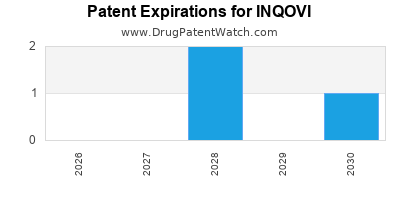

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for INQOVI
Generic Entry Date for INQOVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for INQOVI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Break Through Cancer | EARLY_PHASE1 |
| Astex Pharmaceuticals, Inc. | EARLY_PHASE1 |
| Lachelle D. Weeks, MD, PhD | EARLY_PHASE1 |
Pharmacology for INQOVI
| Drug Class | Nucleoside Metabolic Inhibitor |
| Mechanism of Action | Nucleic Acid Synthesis Inhibitors |
US Patents and Regulatory Information for INQOVI
INQOVI is protected by eleven US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of INQOVI is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,268,800.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for INQOVI
When does loss-of-exclusivity occur for INQOVI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08312435
Patent: 2 ' -flu0r0-2 ' -deoxytetrahydrouridines as cytidine deaminase inhibitors
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 48374
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0818672
Patent: 2'-fluor-2'-desoxitetra-hidrouridinas como inibidores da citidina desaminase
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 02274
Patent: CERTAINS COMPOSES, COMPOSITIONS ET PROCEDES (2 ' -FLU0R0-2 ' -DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1827856
Patent: 2 ' -flu0r0-2 ' -deoxytetrahydrouridines as cytidine deaminase inhibitors
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 70330
Patent: 2-FLUORO-2`-DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 427
Patent: 2-FLUORO-2 - DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120419
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12781
Estimated Expiration: ⤷ Get Started Free
Patent: 23028
Estimated Expiration: ⤷ Get Started Free
Patent: 23029
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 07786
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010095
Patent: 2'-FLUORO-2'-DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8757
Patent: 2'-ФТОР-2'-ДЕЗОКСИТЕТРАГИДРОУРИДИНЫ В КАЧЕСТВЕ ИНГИБИТОРОВ ЦИТИДИНДЕАМИНАЗЫ (2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1000642
Patent: 2'-ФТОР-2'-ДЕЗОКСИТЕТРАГИДРОУРИДИНЫ В КАЧЕСТВЕ ИНГИБИТОРОВ ЦИТИДИНДЕАМИНАЗЫ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 07786
Patent: 2'-FLUORO-2'-DÉSOXYTETRAHYDROURIDINES COMME INHIBITEURS DE CYTIDINE DÉSAMINASE (2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Patent: 47272
Patent: 2'-FLUORO-2'-DEOXYTÉTRAHYDROURIDINES EN TANT QU'INHIBITEURS DE DÉSAMINASE DE LA CYTIDINE (2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0230039
Estimated Expiration: ⤷ Get Started Free
Patent: 0230040
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1051
Estimated Expiration: ⤷ Get Started Free
Patent: C1052
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1000088
Patent: 2 -FLUORO-2 ́DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 46410
Patent: 2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 300044
Estimated Expiration: ⤷ Get Started Free
Patent: 300045
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 4732
Patent: @@@@@-דיפלואורו-@@-דאוקסי@טטראהידרו אורידינים, תכשירים המכילים אותם והשימוש בהם להכנת@תרופות (2',2'-difluoro-2'-deoxytetrahydrouridine, compositions comprising the same and uses thereof in the preparation of medicaments)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 96899
Estimated Expiration: ⤷ Get Started Free
Patent: 59588
Estimated Expiration: ⤷ Get Started Free
Patent: 11500713
Estimated Expiration: ⤷ Get Started Free
Patent: 14177455
Patent: 2'-FLUORO-2'-DEOXYTETRAHYDROURIDINE AS CYTIDINE DEAMINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 78
Patent: مركبات وتركيبات وطرق معينة (Certain Compounds, Compositions and Methods)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7970
Patent: 2'-FLUORO-2'DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10004109
Patent: 2'-FLUORO-2'-DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA. (2 ' -FLU0R0-2 ' -DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 997
Patent: 2'-FLUOR-2 ' -DEOKSITETRAHIDROURIDINI KAO INHIBITORI CITIDIN DEAMINAZE (2 ' -FLU0R0-2 ' -DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1256
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4229
Patent: 2 ' -FLU0R0-2 ' -DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1000055
Patent: 2' - FLUORO - 2' - DESOXITETRAHIDROURIDINAS COMO INHIBIDORES DE CITIDINA DESAMINASA.
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 23047
Estimated Expiration: ⤷ Get Started Free
Patent: 23048
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 07786
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 07786
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 290661
Patent: 2' - فلورو -2' - ديوكسي تترا هيدرو يوريدين كمثبطات سيتيدين دياميناز (2'-Fluoro-2' Deoxytetrahydrouridines as Cytidine Deaminase Inhibitors)
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 323
Patent: 2'-FLUORO-2'-DEOKSITETRAHIDROURIDINI KAO INHIBITORI CITIDIN DEAMINAZE (2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 07786
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1002178
Patent: 2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1543049
Estimated Expiration: ⤷ Get Started Free
Patent: 100091978
Patent: 2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 84011
Estimated Expiration: ⤷ Get Started Free
Patent: 16566
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 45539
Estimated Expiration: ⤷ Get Started Free
Patent: 0924786
Patent: Certain compounds, compositions and methods
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 476
Patent: 2'-ФТОР-2'-ДЕОКСИТЕТРАГІДРОУРИДИНИ ЯК ІНГІБІТОРИ ЦИТИДИНДЕАМІНАЗИ[2'-ФТОР-2'-ДЕОКСИТЕТРАГИДРОУРИДИНЫ КАК ИНГИБИТОРЫ ЦИТИДИНДЕАМИНАЗЫ (2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering INQOVI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Philippines | 12022552250 | ⤷ Get Started Free | |
| Mexico | 2022010214 | ⤷ Get Started Free | |
| South Africa | 201002178 | 2'-FLUORO-2'-DEOXYTETRAHYDROURIDINES AS CYTIDINE DEAMINASE INHIBITORS | ⤷ Get Started Free |
| Canada | 3163122 | ⤷ Get Started Free | |
| European Patent Office | 2447272 | ⤷ Get Started Free | |
| Lithuania | 4069254 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for INQOVI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2207786 | 202340031 | Slovenia | ⤷ Get Started Free | PRODUCT NAME: COMBINATION OF CEDAZURIDINE OR ITS PHARMACEUTICALLY ACCEPTEBLE SALT AND DECITABINE; NATIONAL AUTHORISATION NUMBER: EU/1/23/1756; DATE OF NATIONAL AUTHORISATION: 20230915; AUTHORITY FOR NATIONAL AUTHORISATION: EU |
| 2207786 | C20230040 | Finland | ⤷ Get Started Free | |
| 2207786 | C20230039 | Finland | ⤷ Get Started Free | |
| 2207786 | 23C1052 | France | ⤷ Get Started Free | PRODUCT NAME: UNE COMPOSITION COMPRENANT: LA CEDAZURIDINE OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES, ET LA DECITABINE; REGISTRATION NO/DATE: EU/1/23/1756 20230918 |
| 2207786 | 2023C/550 | Belgium | ⤷ Get Started Free | PRODUCT NAME: CEDAZURIDINE, OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; AUTHORISATION NUMBER AND DATE: EU/1/23/1756 20230918 |
| 2207786 | 2023C/551 | Belgium | ⤷ Get Started Free | PRODUCT NAME: UNE COMPOSITION COMPRENANT : DE LA CEDAZURIDINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI ; ET LA DECITABINE; AUTHORISATION NUMBER AND DATE: EU/1/23/1756 20230918 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for INQOVI
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
