INGREZZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ingrezza, and when can generic versions of Ingrezza launch?
Ingrezza is a drug marketed by Neurocrine and is included in two NDAs. There are twenty-two patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and seventy-two patent family members in thirty-six countries.
The generic ingredient in INGREZZA is valbenazine tosylate. One supplier is listed for this compound. Additional details are available on the valbenazine tosylate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Ingrezza
A generic version of INGREZZA was approved as valbenazine tosylate by LUPIN LTD on April 5th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for INGREZZA?
- What are the global sales for INGREZZA?
- What is Average Wholesale Price for INGREZZA?
Summary for INGREZZA
| International Patents: | 272 |
| US Patents: | 22 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 50 |
| Clinical Trials: | 7 |
| Patent Applications: | 297 |
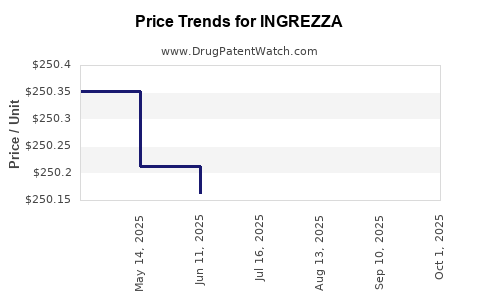
| Drug Prices: | Drug price information for INGREZZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for INGREZZA |
| What excipients (inactive ingredients) are in INGREZZA? | INGREZZA excipients list |
| DailyMed Link: | INGREZZA at DailyMed |
Recent Clinical Trials for INGREZZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Stephen Ruedrich | Phase 4 |
| Neurocrine Biosciences | Phase 4 |
| Michael Bloch | Phase 2 |
Pharmacology for INGREZZA
| Drug Class | Vesicular Monoamine Transporter 2 Inhibitor |
| Mechanism of Action | Vesicular Monoamine Transporter 2 Inhibitors |
Paragraph IV (Patent) Challenges for INGREZZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| INGREZZA | Capsules | valbenazine tosylate | 60 mg | 209241 | 1 | 2022-02-14 |
| INGREZZA | Capsules | valbenazine tosylate | 40 mg and 80 mg | 209241 | 4 | 2021-04-12 |
US Patents and Regulatory Information for INGREZZA
INGREZZA is protected by thirty-three US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-002 | Oct 4, 2017 | AB | RX | Yes | Yes | 8,357,697 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-002 | Oct 4, 2017 | AB | RX | Yes | Yes | 10,906,903 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | |
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-003 | Apr 23, 2021 | RX | Yes | No | 10,912,771 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Neurocrine | INGREZZA | valbenazine tosylate | CAPSULE;ORAL | 209241-001 | Apr 11, 2017 | AB | RX | Yes | No | 10,919,892 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | |
| Neurocrine | INGREZZA SPRINKLE | valbenazine tosylate | CAPSULE;ORAL | 218390-002 | Apr 30, 2024 | RX | Yes | No | 10,857,137 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for INGREZZA
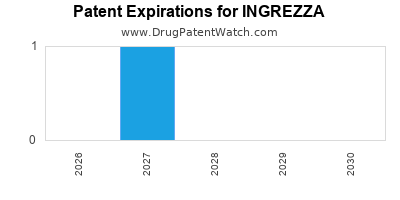
When does loss-of-exclusivity occur for INGREZZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2819
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 18335259
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2020005373
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 76000
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1372567
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0250561
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2090809
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 71873
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3300
Patent: פורמולציית ולבנאזין במינון גבוה ותכשירים, שיטות וערכות קשורות להן (High dosage valbenazine formulation and compositions, methods, and kits related thereto)
Estimated Expiration: ⤷ Get Started Free
Patent: 1770
Patent: פורמולציית ולבנאזין במינון גבוה ותכשירים, שיטות וערכות קשורות להן (High dosage valbenazine formulation and compositions, methods, and kits related thereto)
Estimated Expiration: ⤷ Get Started Free
Patent: 9802
Patent: פורמולציית ולבנאזין במינון גבוה ותכשירים, שיטות וערכות קשורות להן (High dosage valbenazine formulation and compositions, methods, and kits related thereto)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 50006
Estimated Expiration: ⤷ Get Started Free
Patent: 20534305
Patent: 高投与量バルベナジン製剤ならびにそれに関連する組成物、方法およびキット
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 20002841
Patent: FORMULACION DE VALBENAZINA DE ALTA DOSIS Y COMPOSICIONES, METODOS Y KITS RELACIONADOS CON LA MISMA. (HIGH DOSAGE VALBENAZINE FORMULATION AND COMPOSITIONS, METHODS, AND KITS RELATED THERETO.)
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 175
Patent: FORMULATION DE VALBENAZINE À DOSAGE ÉLEVÉ ET COMPOSITIONS, PROCÉDÉS ET KITS ASSOCIÉS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 02500305
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 714
Patent: FORMULACIJA VISOKE DOZE VALBENAZINA I SASTAVI, POSTUPCI I KOMPLETI POVEZANI SA NJIM (HIGH DOSAGE VALBENAZINE FORMULATION AND COMPOSITIONS, METHODS, AND KITS RELATED THERETO)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 84333
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 24958
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 75681
Estimated Expiration: ⤷ Get Started Free
Patent: 1919622
Patent: High dosage VALBENAZINE formulation and compositions, methods, and kits related thereto
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering INGREZZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Jordan | P20200336 | ⤷ Get Started Free | |
| South Korea | 20200066661 | 특정 VMAT2 억제제의 투여 방법 | ⤷ Get Started Free |
| Spain | 2911351 | ⤷ Get Started Free | |
| Japan | 2022040408 | ⤷ Get Started Free | |
| Slovenia | 3394057 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for INGREZZA (Valbenazine)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
