IMBRUVICA Drug Patent Profile
✉ Email this page to a colleague
When do Imbruvica patents expire, and when can generic versions of Imbruvica launch?
Imbruvica is a drug marketed by Pharmacyclics Llc and is included in three NDAs. There are fifty-six patents protecting this drug and four Paragraph IV challenges.
This drug has four hundred and eighty-two patent family members in forty-nine countries.
The generic ingredient in IMBRUVICA is ibrutinib. There are sixteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ibrutinib profile page.
DrugPatentWatch® Generic Entry Outlook for Imbruvica
Imbruvica was eligible for patent challenges on November 13, 2017.
There have been twenty patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are six tentative approvals for the generic drug (ibrutinib), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for IMBRUVICA?
- What are the global sales for IMBRUVICA?
- What is Average Wholesale Price for IMBRUVICA?
Summary for IMBRUVICA
| International Patents: | 482 |
| US Patents: | 56 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 76 |
| Clinical Trials: | 151 |
| Patent Applications: | 3,873 |
| Drug Prices: | Drug price information for IMBRUVICA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for IMBRUVICA |
| What excipients (inactive ingredients) are in IMBRUVICA? | IMBRUVICA excipients list |
| DailyMed Link: | IMBRUVICA at DailyMed |

Recent Clinical Trials for IMBRUVICA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Peter MacCallum Cancer Centre, Australia | Phase 2 |
| Oncternal Therapeutics, Inc | Phase 3 |
| Academic and Community Cancer Research United | Phase 2 |
Pharmacology for IMBRUVICA
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Paragraph IV (Patent) Challenges for IMBRUVICA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| IMBRUVICA | Capsules | ibrutinib | 70 mg | 205552 | 1 | 2018-12-14 |
| IMBRUVICA | Tablets | ibrutinib | 280 mg and 420 mg | 210563 | 1 | 2018-12-14 |
| IMBRUVICA | Tablets | ibrutinib | 560 mg | 210563 | 1 | 2018-11-05 |
| IMBRUVICA | Capsules | ibrutinib | 140 mg | 205552 | 8 | 2017-11-13 |
US Patents and Regulatory Information for IMBRUVICA
IMBRUVICA is protected by eighty-seven US patents and eight FDA Regulatory Exclusivities.
EU/EMA Drug Approvals for IMBRUVICA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International NV | Imbruvica | ibrutinib | EMEA/H/C/003791IMBRUVICA as a single agent is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).IMBRUVICA as a single agent or in combination with rituximab or obinutuzumab or venetoclax is indicated for the treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) (see section 5.1).IMBRUVICA as a single agent or in combination with bendamustine and rituximab (BR) is indicated for the treatment of adult patients with CLL who have received at least one prior therapy.IMBRUVICA as a single agent is indicated for the treatment of adult patients with Waldenström’s macroglobulinaemia (WM) who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo immunotherapy. IMBRUVICA in combination with rituximab is indicated for the treatment of adult patients with WM. | Authorised | no | no | no | 2014-10-21 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for IMBRUVICA
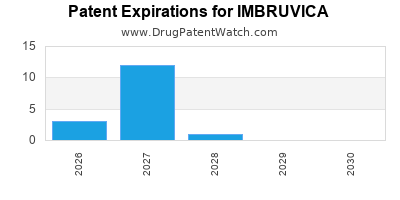
When does loss-of-exclusivity occur for IMBRUVICA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2844
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 3832
Patent: FORMULACIONES FARMACÉUTICAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 8108
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 13271918
Patent: Crystalline forms of a bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 16226279
Patent: Pharmaceutical formulations of Bruton's tyrosine kinase inhibtor
Estimated Expiration: ⤷ Get Started Free
Patent: 16250445
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 18211201
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 18211216
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 20239751
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 21240244
Patent: Pharmaceutical formulations of Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 23200435
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 23202671
Patent: Pharmaceutical formulations of Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 25205154
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014030424
Patent: formas cristalinas de um inibidor de quinase de tirosina de bruton
Estimated Expiration: ⤷ Get Started Free
Patent: 2017018931
Patent: formulações farmacêuticas de um inibidor de tirosina quinase de bruton
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 75986
Patent: FORMES CRISTALLINES D'UN INHIBITEUR DE TYROSINE KINASE DE BRUTON (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 76695
Patent: FORMULATIONS PHARMACEUTIQUES D'INHIBITEUR DE LA TYROSINE KINASE DE BRUTON (PHARMACEUTICAL FORMULATIONS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 91994
Patent: FORMULATION PHARMACEUTIQUE POUR ADMINISTRATION ORALE COMPRENANT DE L'IBRUTINIB (PHARMACEUTICAL FORMULATION FOR ORAL ADMINISTRATION COMPRISING IBRUTINIB)
Estimated Expiration: ⤷ Get Started Free
Patent: 15208
Patent: FORMES CRISTALLINES D'UN INHIBITEUR DE TYROSINE KINASE DE BRUTON (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 77990
Patent: FORMULATIONS PHARMACEUTIQUES D'INHIBITEUR DE LA TYROSINE KINASE DE BRUTON (PHARMACEUTICAL FORMULATIONS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 77995
Patent: FORMULATIONS PHARMACEUTIQUES D'INHIBITEUR DE LA TYROSINE KINASE DE BRUTON (PHARMACEUTICAL FORMULATIONS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 18491
Patent: FORMES CRISTALLINES D'UN INHIBITEUR DE TYROSINE KINASE DE BRUTON (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14003306
Patent: Preparacion farmaceutica oral que comprende un inhibidor de tirosina quinasa de bruton y forma cristalina a del mismo.
Estimated Expiration: ⤷ Get Started Free
Patent: 17000371
Patent: Formas cristalinas de un inhibidor de tirosina quinasa de bruton
Estimated Expiration: ⤷ Get Started Free
Patent: 17003496
Patent: Formas cristalinas de un inhibidor de tirosina quinasa de bruton.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4736178
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 7427498
Patent: 布鲁顿氏酪氨酸激酶抑制剂的医药配方 (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 0354132
Patent: 布鲁顿酪氨酸激酶抑制剂的晶形 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 40408
Patent: Formas cristalinas de un inhibidor de tirosina quinasa de bruton
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 140558
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 014000274
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 017000152
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 022000096
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 14033163
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 19015794
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1492082
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ ИНГИБИТОРА ТИРОЗИНКИНАЗЫ БРУТОНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 54859
Patent: FORMES CRISTALLINES D'UN INHIBITEUR DE TYROSINE KINASE DE BRUTON (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 65084
Patent: FORMULATIONS PHARMACEUTIQUES D'INHIBITEUR DE LA TYROSINE KINASE DE BRUTON (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR)
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1400281
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 08803
Patent: 布魯頓酪氨酸激酶抑制劑的晶形 (CRYSTALLINE FORMS OF A BRUTONS TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 49015
Patent: 布魯頓氏酪氨酸激酶抑制劑的醫藥配方 (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5894
Patent: צורות גבישיות של מעכב ברוטון טירוסין קינאז (Crystalline forms of a bruton's tyrosine kinase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Patent: 3995
Patent: פורמולציות רוקחיות של מעכב ברוטון טירוסין קינאז (Pharmaceutical formulations of bruton's tyrosine kinase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Patent: 2367
Patent: צורות גבישיות של מעכב ברוטון טירוסין קינאז (Crystalline forms of a bruton's tyrosine kinase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Patent: 3284
Patent: פורמולציות רוקחיות של מעכב ברוטון טירוסין קינאז (Pharmaceutical formulations of bruton's tyrosine kinase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Patent: 5394
Patent: צורות גבישיות של מעכב ברוטון טירוסין קינאז (Crystalline forms of a bruton's tyrosine kinase inhibitor)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 36071
Estimated Expiration: ⤷ Get Started Free
Patent: 37086
Estimated Expiration: ⤷ Get Started Free
Patent: 49076
Estimated Expiration: ⤷ Get Started Free
Patent: 15518885
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態
Estimated Expiration: ⤷ Get Started Free
Patent: 18012710
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 18048182
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 18507200
Patent: ブルトン型チロシンキナーゼ阻害剤の薬学的製剤
Estimated Expiration: ⤷ Get Started Free
Patent: 19203008
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 20015744
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 21183617
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 21193095
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 22141701
Patent: ブルトン型チロシンキナーゼ阻害剤の薬学的製剤
Estimated Expiration: ⤷ Get Started Free
Patent: 23162162
Patent: ブルトン型チロシンキナーゼ阻害剤の結晶形態 (CRYSTALLINE FORMS OF BRUTON TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0200222
Patent: أشكال كريستالين لمثبط أنزيم كيناز تيروسين بروتون (CRYSTALLINE FORMS OF A BRUTON’S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 54
Patent: أشكال كريستالين لمثبط أنزيم كيناز تيروسين بروتون (CRYSTALLINE FORMS OF A BRUTON’S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7999
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 4911
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 8290
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON. (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 7669
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON. (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 14014848
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON. (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 17011270
Patent: COMPOSICIONES FARMACEUTICAS DE UN INHIBIDOR DE TIROSINA CINASA DE BRUTON. (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21012478
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON. (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21015368
Patent: COMPOSICIONES FARMACEUTICAS DE UN INHIBIDOR DE TIROSINA CINASA DE BRUTON. (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21015370
Patent: COMPOSICIONES FARMACEUTICAS DE UN INHIBIDOR DE TIROSINA CINASA DE BRUTON. (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21015405
Patent: COMPOSICIONES FARMACEUTICAS DE UN INHIBIDOR DE TIROSINA CINASA DE BRUTON. (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21015408
Patent: COMPOSICIONES FARMACEUTICAS DE UN INHIBIDOR DE TIROSINA CINASA DE BRUTON. (PHARMACEUTICAL FORMULATIONS OF BRUTON'S TYROSINE KINASE INHIBTOR.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 711
Patent: Formes cristallines d'un inhibiteur de tyrosine kinase de bruton
Estimated Expiration: ⤷ Get Started Free
Patent: 643
Patent: FORMULATIONS PHARMACEUTIQUES D'INHIBITEUR DE LA TYROSINE KINASE DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 654
Patent: Formes cristallines d'un inhibiteur de tyrosine kinase de bruton
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2548
Patent: Crystalline forms of a bruton’s tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 3828
Patent: Crystalline forms of a bruton’s tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 1932
Patent: Crystalline forms of a bruton’s tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 4446
Estimated Expiration: ⤷ Get Started Free
Patent: 7725
Patent: Crystalline forms of a bruton’s tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 150174
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 190390
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 241581
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014502681
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 020500028
Patent: CRYSTALLINE FORMS OF A BRUTON`S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 021552282
Patent: CRYSTALLINE FORMS OF A BRUTON`S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 17133990
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 202101389T
Patent: CRYSTALLINE FORMS OF A BRUTON’S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 202102078V
Estimated Expiration: ⤷ Get Started Free
Patent: 201408067Y
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 201707122Q
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1907661
Estimated Expiration: ⤷ Get Started Free
Patent: 2105174
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 2105175
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 2303227
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 2502152
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 150015021
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Patent: 170091785
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 170122220
Patent: 브루톤 타이로신 키나제 저해제의 약제학적 제제
Estimated Expiration: ⤷ Get Started Free
Patent: 190040370
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 200017549
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 210033067
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 220093389
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 230170108
Patent: 브루톤 타이로신 키나아제 저해제의 결정 형태 (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 32912
Estimated Expiration: ⤷ Get Started Free
Patent: 53041
Estimated Expiration: ⤷ Get Started Free
Patent: 62963
Estimated Expiration: ⤷ Get Started Free
Patent: 62964
Estimated Expiration: ⤷ Get Started Free
Patent: 33027
Estimated Expiration: ⤷ Get Started Free
Patent: 10588
Estimated Expiration: ⤷ Get Started Free
Patent: 1402122
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 1636023
Estimated Expiration: ⤷ Get Started Free
Patent: 1811334
Patent: Crystalline forms of a bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 1902483
Estimated Expiration: ⤷ Get Started Free
Patent: 1906612
Patent: Crystalline forms of a Bruton's tyrosine kinase inhibitor
Estimated Expiration: ⤷ Get Started Free
Patent: 2211924
Estimated Expiration: ⤷ Get Started Free
Patent: 2315634
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 14000492
Patent: CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4421
Patent: КРИСТАЛІЧНА ФОРМА ІНГІБІТОРУ ТИРОЗИНКІНАЗИ БРУТОНА (CRYSTALLINE FORMS OF A BRUTON'S TYROSINE KINASE INHIBITOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 6959
Patent: ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ, ЩО МІСТИТЬ ІНГІБІТОР ТИРОЗИНКІНАЗИ БРУТОНА
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 848
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Patent: 740
Patent: FORMAS CRISTALINAS DE UN INHIBIDOR DE TIROSINA QUINASA DE BRUTON
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering IMBRUVICA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Ukraine | 126959 | ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ, ЩО МІСТИТЬ ІНГІБІТОР ТИРОЗИНКІНАЗИ БРУТОНА | ⤷ Get Started Free |
| Australia | 2012205166 | ⤷ Get Started Free | |
| Serbia | 65234 | ⤷ Get Started Free | |
| Australia | 2022202686 | Methods of treating and preventing graft versus host disease | ⤷ Get Started Free |
| Canada | 2991994 | FORMULATION PHARMACEUTIQUE POUR ADMINISTRATION ORALE COMPRENANT DE L'IBRUTINIB (PHARMACEUTICAL FORMULATION FOR ORAL ADMINISTRATION COMPRISING IBRUTINIB) | ⤷ Get Started Free |
| Slovenia | 2526933 | ⤷ Get Started Free | |
| Slovenia | 2526933 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for IMBRUVICA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2201840 | C 2015 016 | Romania | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIB SAU O SARE FARMACEUTIC ACCEPTABILA A ACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/14/945; DATE OF NATIONAL AUTHORISATION: 20141021; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/945; DATE OF FIRST AUTHORISATION IN EEA: 20141021 |
| 2526934 | 132016000119245 | Italy | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIB O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE PER IL TRATTAMENTO DI PAZIENTI ADULTI CON LEUCEMIA LINFOCITICA CRONICA (CLL) PRECEDENTEMENTE NON TRATTATA(IMBRUVICA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/14/945, 20160530 |
| 2526934 | C20160038 00313 | Estonia | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIIB;REG NO/DATE: EU/1/14/945 30.05.2016 |
| 2529621 | 132017000036530 | Italy | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIB O SUO SALE FARMACEUTICAMENTE ACCETTABILE(IMBRUVICA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/4/945, 20150707 |
| 2201840 | C300728 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT ERVAN; REGISTRATION NO/DATE: EU/1/14/945 20141021 |
| 2529621 | PA2017009 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIBAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/14/945 C(2015)4704 20170703 |
| 2526934 | 273 5026-2016 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: IBRUTINIB VO VSETKYCH FORMACH CHRANENYCH ZA- KLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/14/945 20160530 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for IMBRUVICA (Ibrutinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
