HEMANGEOL Drug Patent Profile
✉ Email this page to a colleague
When do Hemangeol patents expire, and what generic alternatives are available?
Hemangeol is a drug marketed by Pierre Fabre Derma and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has forty-seven patent family members in thirty-one countries.
The generic ingredient in HEMANGEOL is propranolol hydrochloride. There are twenty-two drug master file entries for this compound. Thirty-nine suppliers are listed for this compound. Additional details are available on the propranolol hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Hemangeol
A generic version of HEMANGEOL was approved as propranolol hydrochloride by INNOGENIX on October 22nd, 1985.
Summary for HEMANGEOL
| International Patents: | 47 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 156 |
| Clinical Trials: | 4 |
| Patent Applications: | 3,873 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for HEMANGEOL |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for HEMANGEOL |
| What excipients (inactive ingredients) are in HEMANGEOL? | HEMANGEOL excipients list |
| DailyMed Link: | HEMANGEOL at DailyMed |

Recent Clinical Trials for HEMANGEOL
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Christiana Care Health Services | Phase 1 |
| Columbia University | Phase 2 |
| University of Missouri-Columbia | Early Phase 1 |
Pharmacology for HEMANGEOL
| Drug Class | beta-Adrenergic Blocker |
| Mechanism of Action | Adrenergic beta-Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for HEMANGEOL
Paragraph IV (Patent) Challenges for HEMANGEOL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| HEMANGEOL | Oral Solution | propranolol hydrochloride | 4.28 mg/mL | 205410 | 1 | 2022-07-21 |
US Patents and Regulatory Information for HEMANGEOL
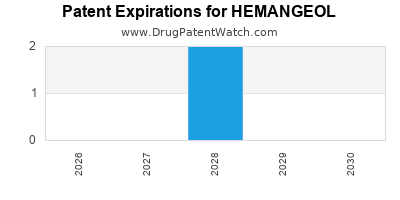
HEMANGEOL is protected by two US patents.
Patents protecting HEMANGEOL
Use of a beta blocker for the manufacture of a medicament for the treatment of hemangiomas
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD TO TREAT HEMANGIOMA.
Use of a beta blocker for the manufacture of a medicament for the treatment of hemangiomas
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD TO TREAT INFANTILE HEMANGIOMA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pierre Fabre Derma | HEMANGEOL | propranolol hydrochloride | SOLUTION;ORAL | 205410-001 | Mar 14, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pierre Fabre Derma | HEMANGEOL | propranolol hydrochloride | SOLUTION;ORAL | 205410-001 | Mar 14, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for HEMANGEOL
See the table below for patents covering HEMANGEOL around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20100087012 | USE OF A BETA BLOCKER FOR THE MANUFACTURE OF A MEDICAMENT FOR THE TREATMENT OF HEMANGIOMAS | ⤷ Try a Trial |
| Australia | 2008313405 | Use of a beta blocker for the manufacture of a medicament for the treatment of hemangiomas | ⤷ Try a Trial |
| New Zealand | 584307 | USE OF A BETA BLOCKER FOR THE MANUFACTURE OF A MEDICAMENT FOR THE TREATMENT OF HEMANGIOMAS | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


