GLYXAMBI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Glyxambi, and when can generic versions of Glyxambi launch?
Glyxambi is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are twelve patents protecting this drug and one Paragraph IV challenge.
This drug has five hundred and sixty-two patent family members in forty-seven countries.
The generic ingredient in GLYXAMBI is empagliflozin; linagliptin. There are twenty-two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the empagliflozin; linagliptin profile page.
DrugPatentWatch® Generic Entry Outlook for Glyxambi
Glyxambi was eligible for patent challenges on May 2, 2015.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 14, 2030. This may change due to patent challenges or generic licensing.
There have been nineteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are three tentative approvals for the generic drug (empagliflozin; linagliptin), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for GLYXAMBI
| International Patents: | 562 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Patent Applications: | 7 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for GLYXAMBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for GLYXAMBI |
| What excipients (inactive ingredients) are in GLYXAMBI? | GLYXAMBI excipients list |
| DailyMed Link: | GLYXAMBI at DailyMed |
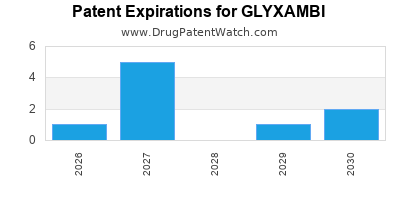
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for GLYXAMBI
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for GLYXAMBI
Anatomical Therapeutic Chemical (ATC) Classes for GLYXAMBI
Paragraph IV (Patent) Challenges for GLYXAMBI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| GLYXAMBI | Tablets | empagliflozin; linagliptin | 10 mg/5 mg and 25 mg/5 mg | 206073 | 9 | 2018-08-01 |
US Patents and Regulatory Information for GLYXAMBI
GLYXAMBI is protected by thirteen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of GLYXAMBI is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting GLYXAMBI
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
DPP IV inhibitor formulations
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR IS >=30 ML/MIN/1.73 M2 AND
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR>=45 ML/MIN/1.73 M2 AND
8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Glucopyranosyl-substituted phenyl derivatives, medicaments containing such compounds, their use and process for their manufacture
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Crystalline form of 1-chloro-4-(.beta.-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy- )-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivate
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Uses of DPP-IV inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Uses of DPP IV inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING TYPE 2 DIABETES MELLITUS BY ADMINISTERING LINAGLIPTIN IN COMBINATION WITH EMPAGLIFLOZIN
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING TYPE 2 DIABETES MELLITUS IN A PATIENT WITH RENAL IMPAIRMENT (45 ML/MIN/1.73 M2
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-001 | Jan 30, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-002 | Jan 30, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-002 | Jan 30, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-001 | Jan 30, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GLYXAMBI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-002 | Jan 30, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-002 | Jan 30, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-001 | Jan 30, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | GLYXAMBI | empagliflozin; linagliptin | TABLET;ORAL | 206073-001 | Jan 30, 2015 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for GLYXAMBI
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim International GmbH | Glyxambi | empagliflozin, linagliptin | EMEA/H/C/003833 Glyxambi, fixed dose combination of empagliflozin and linagliptin, is indicated in adults aged 18 years and older with type 2 diabetes mellitus:to improve glycaemic control when metformin and/or sulphonylurea (SU) and one of the monocomponents of Glyxambi do not provide adequate glycaemic control;when already being treated with the free combination of empagliflozin and linagliptin. |
Authorised | no | no | no | 2016-11-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for GLYXAMBI
When does loss-of-exclusivity occur for GLYXAMBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7970
Estimated Expiration: ⤷ Sign Up
Patent: 7657
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 08288407
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0815331
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 96558
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 08002427
Estimated Expiration: ⤷ Sign Up
China
Patent: 1784270
Estimated Expiration: ⤷ Sign Up
Patent: 4288166
Estimated Expiration: ⤷ Sign Up
Patent: 4353077
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 51239
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0170022
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 18308
Estimated Expiration: ⤷ Sign Up
Patent: 17017
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 87879
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 109977
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 8608
Estimated Expiration: ⤷ Sign Up
Patent: 1000321
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 87879
Estimated Expiration: ⤷ Sign Up
Patent: 98152
Estimated Expiration: ⤷ Sign Up
Patent: 06156
Estimated Expiration: ⤷ Sign Up
Patent: 39577
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 01721
Estimated Expiration: ⤷ Sign Up
Patent: 03351
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 30158
Estimated Expiration: ⤷ Sign Up
Patent: 700020
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 2886
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 95914
Estimated Expiration: ⤷ Sign Up
Patent: 10535850
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 187879
Estimated Expiration: ⤷ Sign Up
Patent: 2017014
Estimated Expiration: ⤷ Sign Up
Patent: 87879
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 2037
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 10001696
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 573
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 612
Estimated Expiration: ⤷ Sign Up
Netherlands
Patent: 0872
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 3242
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 17020
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 090938
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 87879
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 87879
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 205
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 87879
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0909105
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1491554
Estimated Expiration: ⤷ Sign Up
Patent: 100049595
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 02748
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 72325
Estimated Expiration: ⤷ Sign Up
Patent: 0914030
Estimated Expiration: ⤷ Sign Up
Patent: 1436798
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 10000073
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 0384
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 296
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering GLYXAMBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Malaysia | 152037 | PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE | ⤷ Sign Up |
| South Korea | 20090009226 | DPP IV INHIBITOR FORMULATIONS | ⤷ Sign Up |
| Japan | 5595914 | ⤷ Sign Up | |
| Mexico | 2007013144 | FORMA CRISTALINA DE 1-CLORO-4-((-D-GLUCOPIRANOS-1-IL)-2-[4-((S)- TETRAHIDROFURAN-3-ILOXI)-BENCIL]-BENCENO, UN METODO PARA SU PREPARACION Y EL USO DEL MISMO PARA PREPARAR MEDICAMENTOS. (CRYSTALLINE FORM OF 1-CHLORO-4-(????-D-GLUCOPYRANOS-1-YL)-2-[4-((S) -TETRAHYDROFURAN-3-YLOXY)-BENZYL]-BENZENE, A METHOD FOR ITS PREPARATION AND THE USE THEREOF FOR PREPARING MEDICAMENTS.) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GLYXAMBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1084705 | CA 2014 00063 | Denmark | ⤷ Sign Up | PRODUCT NAME: SITAGLIPTIN OG FARMACEUTISK SALTE DERAF, HERUNDER SITAGLIPTIN PHOSPHAT MONOHYDRAT; REG. NO/DATE: EU/1/07/383/001-024 AND EU/1/07/382/001-024 20070321 |
| 1084705 | PA2014042 | Lithuania | ⤷ Sign Up | PRODUCT NAME: VILDAGLIPTINUM; REGISTRATION NO/DATE: EU/1/07/414/001-010, 2007 09 26 EU/1/07/414/018 20070926 |
| 1084705 | C01084705/04 | Switzerland | ⤷ Sign Up | PRODUCT NAME: LINAGLIPTIN; REGISTRATION NO/DATE: SWISSMEDIC 61893 08.03.2012 |
| 1532149 | 118 5013-2011 | Slovakia | ⤷ Sign Up | PRODUCT NAME: LINAGLIPTIN; REGISTRATION NO/DATE: EU/1/11/707/001 - EU/1/11/707/011 20110824 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


