GIVLAARI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Givlaari, and what generic alternatives are available?
Givlaari is a drug marketed by Alnylam Pharms Inc and is included in one NDA. There are twelve patents protecting this drug.
This drug has three hundred and twenty-two patent family members in forty-three countries.
The generic ingredient in GIVLAARI is givosiran sodium. One supplier is listed for this compound. Additional details are available on the givosiran sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Givlaari
Givlaari was eligible for patent challenges on November 20, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 3, 2034. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for GIVLAARI
| International Patents: | 322 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for GIVLAARI |
| What excipients (inactive ingredients) are in GIVLAARI? | GIVLAARI excipients list |
| DailyMed Link: | GIVLAARI at DailyMed |
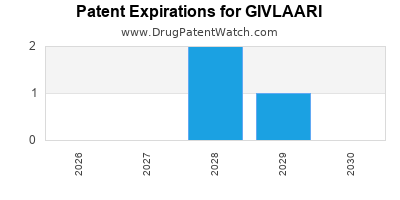

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for GIVLAARI
Generic Entry Date for GIVLAARI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for GIVLAARI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Alnylam Pharmaceuticals | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for GIVLAARI
US Patents and Regulatory Information for GIVLAARI
GIVLAARI is protected by twelve US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of GIVLAARI is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting GIVLAARI
Compositions and methods for inhibiting expression of the ALAS1 gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Compositions and methods for inhibiting expression of the ALAS1 gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Glycoconjugates of RNA interference agents
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for inhibiting expression of the ALAS1 gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Carbohydrate conjugates as delivery agents for oligonucleotides
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Carbohydrate conjugates as delivery agents for oligonucleotides
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Compositions and methods for inhibiting expression of the ALAS1 gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Compositions comprising alternating 2'-modified nucleosides for use in gene modulation
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for inhibiting expression of the ALAS1 gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ACUTE HEPATIC PORPHYRIA
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting GIVLAARI
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF ADULTS WITH ACUTE HEPATIC PORPHYRIA (AHP)
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GIVLAARI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for GIVLAARI
When does loss-of-exclusivity occur for GIVLAARI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7920
Estimated Expiration: ⤷ Try a Trial
Patent: 8658
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 14331604
Estimated Expiration: ⤷ Try a Trial
Patent: 20286311
Estimated Expiration: ⤷ Try a Trial
Patent: 23266354
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2016007226
Estimated Expiration: ⤷ Try a Trial
Patent: 2020001264
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 25357
Estimated Expiration: ⤷ Try a Trial
Patent: 27061
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 16000772
Estimated Expiration: ⤷ Try a Trial
Patent: 18000158
Estimated Expiration: ⤷ Try a Trial
China
Patent: 5980559
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 160195
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0200822
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 016000073
Estimated Expiration: ⤷ Try a Trial
Patent: 022000085
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 6477
Estimated Expiration: ⤷ Try a Trial
Patent: 1690685
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
Patent: 93463
Estimated Expiration: ⤷ Try a Trial
Guatemala
Patent: 1600066
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 21738
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 49227
Estimated Expiration: ⤷ Try a Trial
Patent: 000034
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 4749
Estimated Expiration: ⤷ Try a Trial
Patent: 2747
Estimated Expiration: ⤷ Try a Trial
Patent: 2726
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 13227
Estimated Expiration: ⤷ Try a Trial
Patent: 89254
Estimated Expiration: ⤷ Try a Trial
Patent: 16539623
Estimated Expiration: ⤷ Try a Trial
Patent: 20096582
Estimated Expiration: ⤷ Try a Trial
Patent: 23120219
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 052628
Estimated Expiration: ⤷ Try a Trial
Patent: 2020527
Estimated Expiration: ⤷ Try a Trial
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 3490
Estimated Expiration: ⤷ Try a Trial
Patent: 7646
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 16004319
Estimated Expiration: ⤷ Try a Trial
Patent: 22001017
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 000
Estimated Expiration: ⤷ Try a Trial
Netherlands
Patent: 1061
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 8995
Estimated Expiration: ⤷ Try a Trial
Patent: 7749
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 161130
Estimated Expiration: ⤷ Try a Trial
Patent: 211249
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 016500574
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201910929Q
Estimated Expiration: ⤷ Try a Trial
Patent: 201602631X
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 52628
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1602931
Estimated Expiration: ⤷ Try a Trial
Patent: 1802919
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2307389
Estimated Expiration: ⤷ Try a Trial
Patent: 2469850
Estimated Expiration: ⤷ Try a Trial
Patent: 160079793
Estimated Expiration: ⤷ Try a Trial
Patent: 210122877
Estimated Expiration: ⤷ Try a Trial
Patent: 220159478
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 04510
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 94080
Estimated Expiration: ⤷ Try a Trial
Patent: 68330
Estimated Expiration: ⤷ Try a Trial
Patent: 1524991
Estimated Expiration: ⤷ Try a Trial
Patent: 2106697
Estimated Expiration: ⤷ Try a Trial
Patent: 2310853
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 16000114
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 4961
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering GIVLAARI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2569036 | COMPOSITIONS CHIMERIQUES OLIGOMERES A BRECHE (CHIMERIC GAPPED OLIGOMERIC COMPOSITIONS) | ⤷ Try a Trial |
| Spain | 2628535 | ⤷ Try a Trial | |
| Germany | 50206993 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GIVLAARI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3052628 | C202030043 | Spain | ⤷ Try a Trial | PRODUCT NAME: GIVOSIRAN O UNA SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/20/1428; DATE OF AUTHORISATION: 20200302; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1428; DATE OF FIRST AUTHORISATION IN EEA: 20200302 |
| 3052628 | 37/2020 | Austria | ⤷ Try a Trial | PRODUCT NAME: GIVOSIRAN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/20/1428 (MITTEILUNG) 20200304 |
| 3052628 | SPC/GB20/042 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: GIVOSIRAN AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/20/1428 (NI) 20200304; UK PLGB 50597/0004 20200304 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


