GILOTRIF Drug Patent Profile
✉ Email this page to a colleague
When do Gilotrif patents expire, and what generic alternatives are available?
Gilotrif is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eighty-six patent family members in forty-six countries.
The generic ingredient in GILOTRIF is afatinib dimaleate. There are six drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the afatinib dimaleate profile page.
DrugPatentWatch® Generic Entry Outlook for Gilotrif
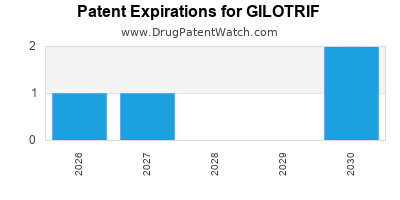
Gilotrif was eligible for patent challenges on July 12, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 19, 2030. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for GILOTRIF
| International Patents: | 186 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 48 |
| Clinical Trials: | 16 |
| Patent Applications: | 666 |
| Drug Prices: | Drug price information for GILOTRIF |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for GILOTRIF |
| What excipients (inactive ingredients) are in GILOTRIF? | GILOTRIF excipients list |
| DailyMed Link: | GILOTRIF at DailyMed |

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for GILOTRIF
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for GILOTRIF
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Centre, Singapore | Phase 1 |
| Vanderbilt-Ingram Cancer Center | Phase 1 |
| Santosh Kesari | Phase 1 |
Pharmacology for GILOTRIF
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for GILOTRIF
Paragraph IV (Patent) Challenges for GILOTRIF
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| GILOTRIF | Tablets | afatinib dimaleate | 20 mg, 30 mg and 40 mg | 201292 | 7 | 2017-07-12 |
US Patents and Regulatory Information for GILOTRIF
GILOTRIF is protected by five US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of GILOTRIF is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting GILOTRIF
Process for drying of BIBW2992, of its salts and of solid pharmaceutical formulations comprising this active ingredient
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Process for preparing amino crotonyl compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid pharmaceutical formulations comprising BIBW 2992
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Quinazoline derivatives for the treatment of cancer diseases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Quinazoline derivatives and pharmaceutical compositions containing them
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting GILOTRIF
FIRST-LINE TREATMENT OF METASTATIC NON-SMALL CELL LUNG CANCER WHOSE TUMORS HAVE NON-RESISTANT EPIDERMAL GROWTH FACTOR (EGFR) MUTATIONS OTHER THAN EXON 19 DELETIONS OR EXON 21 (L858R) SUBSTITUTION MUTATIONS AS DETECTED BY AN FDA-APPROVED TEST
Exclusivity Expiration: ⤷ Sign Up
REVISIONS TO THE PEDIATRIC USE SUBSECTION OF LABELING TO INCLUDE THE RESULTS FROM CLINICAL STUDY 1200.120, CONDUCTED TO FULFILL A PEDIATRIC WRITTEN REQUEST
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-001 | Jul 12, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-002 | Jul 12, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-003 | Jul 12, 2013 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-001 | Jul 12, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-001 | Jul 12, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-002 | Jul 12, 2013 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GILOTRIF
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-001 | Jul 12, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-003 | Jul 12, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | GILOTRIF | afatinib dimaleate | TABLET;ORAL | 201292-002 | Jul 12, 2013 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for GILOTRIF
When does loss-of-exclusivity occur for GILOTRIF?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2062
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 09254574
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0913379
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 26472
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 10001275
Estimated Expiration: ⤷ Sign Up
China
Patent: 2056589
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 80463
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0161061
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 17895
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 99971
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 10010650
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 2168
Estimated Expiration: ⤷ Sign Up
Patent: 1001852
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 99971
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 52478
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 29863
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 9054
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 32367
Estimated Expiration: ⤷ Sign Up
Patent: 11522011
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 1240
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 10012939
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 478
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 030
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 9568
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 100252
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 99971
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 99971
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 943
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 99971
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1007805
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1641517
Estimated Expiration: ⤷ Sign Up
Patent: 110025908
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 88031
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 53203
Estimated Expiration: ⤷ Sign Up
Patent: 1000472
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 10000557
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 2549
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 867
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering GILOTRIF around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Croatia | P20131214 | ⤷ Sign Up | |
| Chile | 2010001275 | Metodo para producir un producto intermedio compactado de bibw 2992 ma2 que comprende una etapa de compactacion por rodillos de bibw 2992 ma2 u opcionalmente en una premezcla con 0-1,0% de lubricante en un mezclador de caida libre o de volteo y una etapa de tamizado; producto intermedio compactado; comprimido. | ⤷ Sign Up |
| Hungary | E029863 | ⤷ Sign Up | |
| Japan | 2011522011 | ⤷ Sign Up | |
| Ukraine | 102549 | СПОСОБ ПОЛУЧЕНИЯ УПЛОТНЕННОГО ПРОМЕЖУТОЧНОГО ПРОДУКТА ДИМАЛЕАТА BIBW 2992, УПЛОТНЕННЫЙ ПРОМЕЖУТОЧНЫЙ ПРОДУКТ И ТВЕРДАЯ ПЕРОРАЛЬНАЯ ТАБЛЕТКА;СПОСІБ ОДЕРЖАННЯ УЩІЛЬНЕНОГО ПРОМІЖНОГО ПРОДУКТУ ДИМАЛЕАТУ BIBW 2992, УЩІЛЬНЕНИЙ ПРОМІЖНИЙ ПРОДУКТ ТА ТВЕРДА ПЕРОРАЛЬНА ТАБЛЕТКА (PROCESS FOR THE PREPARATION OF COMPACTED INTERMEDIATE COMPRISING BIBW 2992 DIMALEATE, COMPACTED INTERMEDIATE AND SOLID DOSAGE FORM) | ⤷ Sign Up |
| Montenegro | 02777 | DERIVATI HINAZOLINA, LEKOVI KOII SADRŽE OVA JEDINJENJA, NJIHOVA PRIMENA I POSTUPAK ZA NJIHOVO PRIPREMANJE (QUINAZOLINE DERIVATIVES, MEDICAMENTS CONTAINING SAID COMPOUNDS, THEIR UTILIZATION AND METHOD FOR THE PRODUCTION THEREOF) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GILOTRIF
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1345910 | C01345910/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: AFATINIB; REGISTRATION NO/DATE: SWISSMEDIC 63042 17.01.2014 |
| 1345910 | CA 2014 00006 | Denmark | ⤷ Sign Up | PRODUCT NAME: AFATINIB, INKLUSIVE TAUTOMERERNE, STEREOISOMERERNE OG SALTENE DERAF, EVENTUELT I FORM AF ET FYSIOLOGISK ACCEPTABELT SALT DERAF, FORTRINSVIS ET MALEATSALT DERAF OG MERE FORETRUKKET ET DIMALEATSALT DERAF; REG. NO/DATE: EU/1/13/879 20130925 |
| 1345910 | 2014C/009 | Belgium | ⤷ Sign Up | PRODUCT NAME: AFATINIB ET SES TAUTOMERES, SES STEREOISOMERES ET SES SELS PHYSIOLOGIQUEMENT ACCEPTABLES AVEC DES ACIDES OU BASES INORGANIQUES OU ORGANIQUES, EN PARTICULIER, AFATINIB SOUS FORME DE SEL DE MALEATE OU DE DIMALEATE; AUTHORISATION NUMBER AND DATE: EU/1/13/879 20130927 |
| 1345910 | 1490011-2 | Sweden | ⤷ Sign Up | PRODUCT NAME: AFATINIB, TAUTOMERER, STEREOISOMERER OCH SALTER DAERAV, FYSIOLOGISKT GODTAGBARA SALTER MED OORGANISKA ELLER ORGANISKA SYROR ELLER BASER, SAERSKILT ETT MALEATSALT DAERAV, MER FOERETRAEDELSEVIS ETT DIMALEATSALT DAERAV; REG. NO/DATE: EU/1/13/879 20130925 |
| 1345910 | C300643 | Netherlands | ⤷ Sign Up | PRODUCT NAME: AFATINIB, DE TAUTOMEREN, STEREOISOMEREN EN ZOUTEN DAARVAN, IN HET BIJZONDER FYSIOLOGISCH AANVAARDBARE ZOUTEN MET ANORGANISCHE OF ORGANISCHE ZUREN OF BASEN, MEER IN HET BIJZONDER ZOUTEN MET MALEINEZUUR, MET NAME EEN DIMALEAATZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/13/879/001-012 20130925 |
| 1345910 | 2014/009 | Ireland | ⤷ Sign Up | PRODUCT NAME: AFATINIB, THE TAUTOMERS, THE STEREOISOMERS AND THE SALTS THEREOF, OPTIONALLY IN THE FORM OF PHYSIOLOGICALLY ACCEPTABLE SALTS THEREOF WITH INORGANIC OR ORGANIC ACIDS OR BASES, PREFERABLY SALTS WITH MALEIC ACID; REGISTRATION NO/DATE: EU/1/13/879/001-012 20130925 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


