GENTAMICIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Gentamicin, and what generic alternatives are available?
Gentamicin is a drug marketed by Intl Medication, Alpharma Us Pharms, Cosette, Fougera Pharms Inc, Padagis Us, Pharmaderm, Taro, Abbott, Eugia Pharma, Fresenius Kabi Usa, Hikma, Hospira, Kalapharm, Pharm Spec, Solopak, Teva Parenteral, Watson Labs, Wyeth Ayerst, Akorn, Fera Pharms Llc, Encube, Alcon Pharms Ltd, Bausch And Lomb, Epic Pharma Llc, Paco, Sandoz, B Braun, and Baxter Hlthcare. and is included in forty-one NDAs.
The generic ingredient in GENTAMICIN is gentamicin sulfate. There are fifteen drug master file entries for this compound. Twenty-six suppliers are listed for this compound. Additional details are available on the gentamicin sulfate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Gentamicin
A generic version of GENTAMICIN was approved as gentamicin sulfate by SANDOZ on December 31st, 1969.
Summary for GENTAMICIN
| US Patents: | 0 |
| Applicants: | 28 |
| NDAs: | 41 |
| Raw Ingredient (Bulk) Api Vendors: | 61 |
| Clinical Trials: | 157 |
| Patent Applications: | 504 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for GENTAMICIN |
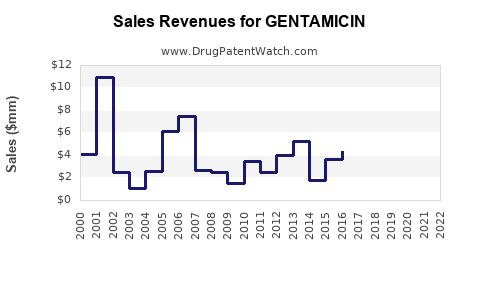
| Drug Sales Revenues: | Drug sales revenues for GENTAMICIN |
| DailyMed Link: | GENTAMICIN at DailyMed |
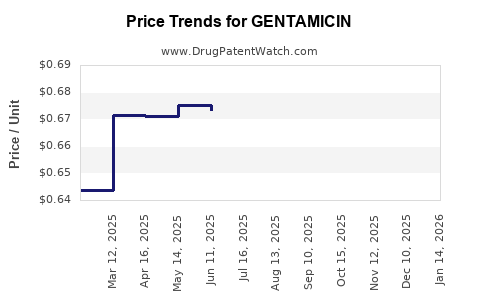
See drug prices for GENTAMICIN
Recent Clinical Trials for GENTAMICIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wake Forest University Health Sciences | Phase 3 |
| HagaZiekenhuis | Phase 3 |
| Leiden University Medical Center | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for GENTAMICIN
US Patents and Regulatory Information for GENTAMICIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baxter Hlthcare | GENTAMICIN SULFATE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | gentamicin sulfate | INJECTABLE;INJECTION | 062373-008 | Sep 7, 1982 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Akorn | GENTAMICIN SULFATE | gentamicin sulfate | OINTMENT;OPHTHALMIC | 064093-001 | Aug 31, 1995 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pharm Spec | GENTAMICIN SULFATE | gentamicin sulfate | INJECTABLE;INJECTION | 062340-001 | Mar 28, 1983 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Hospira | GENTAMICIN SULFATE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | gentamicin sulfate | INJECTABLE;INJECTION | 062414-004 | Aug 15, 1983 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Taro | GENTAMICIN SULFATE | gentamicin sulfate | OINTMENT;TOPICAL | 062477-001 | Dec 23, 1983 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


