GEMCITABINE Drug Patent Profile
✉ Email this page to a colleague
When do Gemcitabine patents expire, and when can generic versions of Gemcitabine launch?
Gemcitabine is a drug marketed by Accord Hlthcare, Actavis Inc, Actavis Totowa, Am Regent, Apotex, Dr Reddys Labs Ltd, Emcure Pharms Ltd, Fresenius Kabi Usa, Gland, Hameln Rds Gmbh, Hikma, Hikma Intl Pharms, Hospira, Hospira Inc, Jiangsu Hansoh Pharm, Meitheal, Mylan Labs Ltd, Novast Labs, Pharmobedient, Sagent Pharms, Sagent Pharms Inc, Shilpa, Sun Pharm, Teva Pharms, and Teyro Labs. and is included in thirty NDAs.
The generic ingredient in GEMCITABINE is gemcitabine hydrochloride. There are thirty drug master file entries for this compound. Sixteen suppliers are listed for this compound. Additional details are available on the gemcitabine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Gemcitabine
A generic version of GEMCITABINE was approved as gemcitabine hydrochloride by HOSPIRA INC on November 15th, 2010.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for GEMCITABINE?
- What are the global sales for GEMCITABINE?
- What is Average Wholesale Price for GEMCITABINE?
Summary for GEMCITABINE
| US Patents: | 0 |
| Applicants: | 25 |
| NDAs: | 30 |
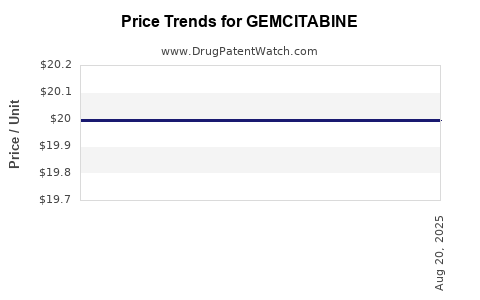
| Drug Prices: | Drug price information for GEMCITABINE |
| What excipients (inactive ingredients) are in GEMCITABINE? | GEMCITABINE excipients list |
| DailyMed Link: | GEMCITABINE at DailyMed |