FLECTOR Drug Patent Profile
✉ Email this page to a colleague
When do Flector patents expire, and when can generic versions of Flector launch?
Flector is a drug marketed by Ibsa and is included in one NDA.
The generic ingredient in FLECTOR is diclofenac epolamine. There are forty-seven drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the diclofenac epolamine profile page.
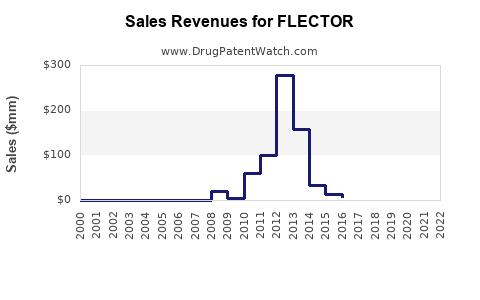
Summary for FLECTOR
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 7 |
| Raw Ingredient (Bulk) Api Vendors: | 25 |
| Clinical Trials: | 5 |
| Patent Applications: | 103 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for FLECTOR |
| What excipients (inactive ingredients) are in FLECTOR? | FLECTOR excipients list |
| DailyMed Link: | FLECTOR at DailyMed |

Recent Clinical Trials for FLECTOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Fidia Farmaceutici s.p.a. | Phase 3 |
| Fidia Farmaceutici s.p.a. | Phase 1 |
| Actavis Inc. | Phase 3 |
Pharmacology for FLECTOR
| Drug Class | Nonsteroidal Anti-inflammatory Drug |
| Mechanism of Action | Cyclooxygenase Inhibitors |
| Physiological Effect | Decreased Prostaglandin Production |
Anatomical Therapeutic Chemical (ATC) Classes for FLECTOR
Paragraph IV (Patent) Challenges for FLECTOR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FLECTOR | Topical Patch | diclofenac epolamine | 1.3% | 021234 | 1 | 2015-06-26 |
US Patents and Regulatory Information for FLECTOR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ibsa | FLECTOR | diclofenac epolamine | SYSTEM;TOPICAL | 021234-001 | Jan 31, 2007 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for FLECTOR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ibsa | FLECTOR | diclofenac epolamine | SYSTEM;TOPICAL | 021234-001 | Jan 31, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Ibsa | FLECTOR | diclofenac epolamine | SYSTEM;TOPICAL | 021234-001 | Jan 31, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for FLECTOR
See the table below for patents covering FLECTOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | H06305958 | PLASTER ANTIPHLOGISTIC ANALGESIC PLASTER FOR EXTERNAL USE | ⤷ Try a Trial |
| Canada | 1303050 | SEL DE DICLOFENAC ET D'UNE BASE ORGANIQUE CYCLIQUE, ET COMPOSITIONS PHARMACEUTIQUES QUI EN CONTIENNENT (SALT OF DICLOFENAC WITH A CYCLIC ORGANIC BASE AND PHARMACEUTICAL COMPOSITIONS WHICH CONTAIN IT) | ⤷ Try a Trial |
| Greece | 3034616 | ⤷ Try a Trial | |
| Spain | 2029254 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


