Last updated: July 29, 2025
Introduction
ESTROGEL® is a topical estrogen therapy primarily used to treat symptoms of vaginal and vulvar atrophy caused by menopause. It features a unique formulation of estradiol in an aqueous gel, allowing localized hormone delivery with minimal systemic absorption. As hormone replacement therapy (HRT) markets evolve, understanding ESTROGEL’s market dynamics and financial prospects becomes essential for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
This analysis dissects the current market landscape, examines key drivers and challenges, explores competitive positioning, and projects ESTROGEL’s financial trajectory over the coming years.
Market Landscape and Demographics
Growing Prevalence of Menopause-Related Disorders
The global aging population drives increased demand for menopause-related therapies. According to the World Health Organization, women aged 50 and above represent a rapidly expanding demographic, with menopause affecting approximately 50% of women worldwide. The incidence of genitourinary syndrome of menopause (GSM)—encompassing vaginal dryness, itching, and dyspareunia—continues to rise, amplifying the need for effective local estrogen products like ESTROGEL.
Preference for Localized Therapies
Patients and clinicians favor localized treatments over systemic HRT due to improved safety profiles, fewer side effects, and targeted symptom relief. ESTROGEL’s formulation aligns well with this trend, positioning it favorably within the therapeutic landscape.
Regulatory Environment
ESTROGEL’s regulatory trajectory in key markets (e.g., FDA in the U.S., EMA in Europe) influences its market access and adoption. Its approval history and ongoing regulatory evaluations shape its commercial prospects.
Key Market Drivers
Differentiation of Delivery Mechanism
ESTROGEL’s water-based topical formulation facilitates efficient hormone absorption with minimal systemic exposure, reducing risks such as breast cancer, cardiovascular events, or thromboembolism—common concerns with systemic HRT. This differentiation could enhance its market appeal among safety-conscious patients.
Clear Clinical Efficacy
Clinical trials confirm ESTROGEL’s effectiveness in alleviating GSM symptoms, supporting clinical adoption and prescriber confidence. Its rapid onset of relief and ease of application are promising factors driving prescriber preference.
Increasing Awareness and Acceptance
Educational initiatives and increased awareness among healthcare providers promote acceptance of localized estrogen therapies. As more providers recognize the benefits of ESTROGEL, market penetration is likely to accelerate.
Reimbursement and Insurance Coverage
Reimbursement policies in developed markets substantially influence product uptake. Positive coverage decisions and inclusion in formularies bolster sales potential.
Market Challenges
Competition from Established and Emerging Therapies
ESTROGEL competes with other local estrogen products, such as vaginal creams, tablets, rings, and suppositories, often offered by major pharmaceutical companies with entrenched market presence. Recently introduced products with novel delivery systems or combined therapies further fragment the market landscape.
Market Penetration and Physician Adoption
Despite clinical efficacy, prescriber familiarity and comfort levels with ESTROGEL influence its growth. Educational barriers and reluctance to switch from familiar regimens can hinder uptake.
Pricing and Cost-Effectiveness
Pricing strategies must balance profitability with accessibility. Insurance reimbursement levels and patient out-of-pocket costs affect demand, especially in price-sensitive markets.
Regulatory Uncertainties
Differences in approval processes, labeling requirements, and post-market surveillance obligations across jurisdictions influence market entry timelines and costs.
Competitive Positioning
Product Differentiation
ESTROGEL’s unique formulation offers safety advantages and ease of application, which can be leveraged in marketing to distinguish it from other estrogen therapies. Its reputation for localized, minimal systemic absorption aligns with healthcare trends favoring safer modalities.
Strategic Partnerships and Alliances
Collaboration with healthcare providers, advocacy groups, or distribution channels can facilitate market access and patient education, crucial for expansion.
Brand Awareness and Market Penetration
Investing in clinical education, physician training, and patient support programs enhances brand recognition. Real-world evidence demonstrating safety and efficacy solidifies its positioning.
Financial Trajectory Projections
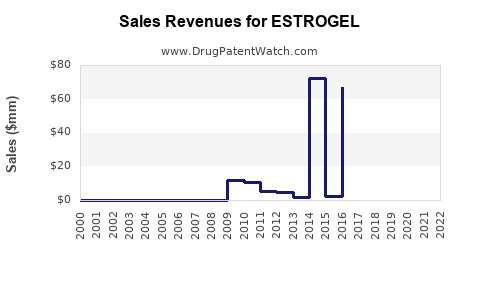
Revenue Growth Outlook
Considering current demographics and product advantages, ESTROGEL’s revenues are poised for steady growth, contingent upon successful market penetration strategies. In mature markets, a compounded annual growth rate (CAGR) of approximately 8-12% over the next five years is plausible, driven by increasing adoption and expanding indications.
Investment and Development Costs
Further clinical trials, regulatory filings, and commercialization efforts require significant upfront and ongoing investments. These costs impact short-term profitability but are critical for long-term market share gains.
Market Expansion Potential
Emerging markets present substantial growth opportunities due to rising awareness, increasing menopause-related healthcare expenditure, and expanding access. Tailored strategies for these regions could compound growth rates further.
Impact of Patent and Exclusivity Status
Patent protection, exclusivity periods, and potential biosimilar or generic entrants influence pricing power and profit margins—factors directly affecting revenue streams.
Future Product Line Extensions
Development of combination formulations, novel delivery systems, or expanded indications (such as osteoporosis or breast health) can diversify revenue streams and enhance financial robustness over time.
Strategic Recommendations
To optimize financial trajectory, stakeholders should focus on:
- Accelerating market access through regulatory approvals,
- Strengthening clinical data and real-world evidence,
- Expanding educational efforts targeting physicians and patients,
- Implementing competitive pricing strategies aligned with reimbursement landscapes,
- Pursuing strategic alliances for distribution and promotion in emerging markets,
- Innovating through formulation improvements or new indications.
Key Takeaways
- Demographic trends support sustained growth for localized estrogen therapy products like ESTROGEL.
- Safety profile and delivery mechanism differentiation are critical competitive advantages.
- Market challenges include stiff competition and prescriber inertia, requiring targeted educational and marketing strategies.
- Financial prospects hinge on successful market penetration, reimbursement support, and geographical expansion.
- Innovation and strategic partnerships will play vital roles in sustaining revenue growth over the medium to long term.
FAQs
1. What sets ESTROGEL apart from other topical estrogen therapies?
Its water-based, topical formulation minimizes systemic absorption while offering effective symptom relief, positioning it as a safer alternative with fewer side effects compared to traditional estrogen creams or rings.
2. In which markets is ESTROGEL most likely to succeed?
Markets with aging populations, high awareness of menopause management, and favorable regulatory environments—such as North America and Europe—present the best initial opportunities, with expanding potential in Asia-Pacific and Latin America.
3. How does reimbursement impact ESTROGEL’s market growth?
Positive reimbursement policies increase patient access, incentivize prescribers, and improve sales. Conversely, lack of coverage or high out-of-pocket costs can slow adoption.
4. What are the primary competitors of ESTROGEL?
Other local estrogen products—including vaginal creams, tablets, rings, and bioidentical hormones—serve as primary competition, especially products with well-established prescriber habits.
5. What future developments could influence ESTROGEL's financial trajectory?
New indications, combination therapies, delivery innovations, and strategic alliances all have the potential to enhance market share and revenue in the coming years.
References
[1] World Health Organization. Menopause and aging demographics factsheet. 2022.
[2] U.S. Food and Drug Administration. ESTROGEL FDA approval documents. 2014.
[3] European Medicines Agency. Product Information: ESTROGEL. 2015.
[4] MarketResearch.com. Global menopause therapy market forecasts, 2023–2028. 2023.
[5] IQVIA. Global Prescription Drug Market Trends Report. 2022.