EPIDUO Drug Patent Profile
✉ Email this page to a colleague
When do Epiduo patents expire, and when can generic versions of Epiduo launch?
Epiduo is a drug marketed by Galderma Labs Lp and Galderma Labs and is included in two NDAs. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-eight patent family members in twenty-five countries.
The generic ingredient in EPIDUO is adapalene; benzoyl peroxide. There are twelve drug master file entries for this compound. Thirteen suppliers are listed for this compound. Additional details are available on the adapalene; benzoyl peroxide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Epiduo
A generic version of EPIDUO was approved as adapalene; benzoyl peroxide by PADAGIS ISRAEL on January 23rd, 2018.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EPIDUO?
- What are the global sales for EPIDUO?
- What is Average Wholesale Price for EPIDUO?
Summary for EPIDUO
| International Patents: | 68 |
| US Patents: | 4 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 31 |
| Patent Applications: | 66 |
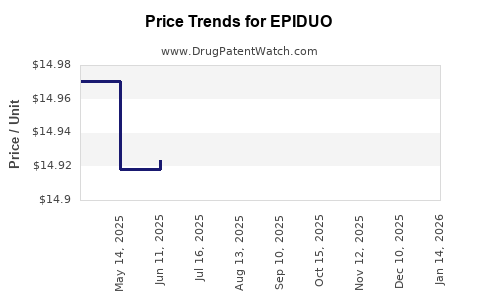
| Drug Prices: | Drug price information for EPIDUO |
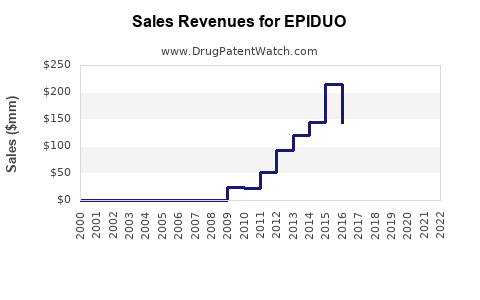
| Drug Sales Revenues: | Drug sales revenues for EPIDUO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EPIDUO |
| What excipients (inactive ingredients) are in EPIDUO? | EPIDUO excipients list |
| DailyMed Link: | EPIDUO at DailyMed |
Recent Clinical Trials for EPIDUO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Bausch Health Americas, Inc. | Phase 2 |
| Mahidol University | Phase 2 |
| Beiersdorf | Phase 2 |
Pharmacology for EPIDUO
| Drug Class | Retinoid |
Paragraph IV (Patent) Challenges for EPIDUO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EPIDUO | Gel | adapalene; benzoyl peroxide | 0.1%/2.5% | 022320 | 1 | 2011-12-30 |
US Patents and Regulatory Information for EPIDUO
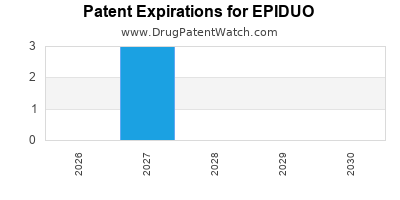
EPIDUO is protected by four US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EPIDUO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| Galderma Labs Lp | EPIDUO | adapalene; benzoyl peroxide | GEL;TOPICAL | 022320-001 | Dec 8, 2008 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for EPIDUO
When does loss-of-exclusivity occur for EPIDUO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1989
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07274288
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0713182
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 56456
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1541320
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 50136
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 14473
Estimated Expiration: ⤷ Get Started Free
Patent: 21398
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 46318
Estimated Expiration: ⤷ Get Started Free
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 46318
Estimated Expiration: ⤷ Get Started Free
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
France
Patent: 03603
Patent: COMBINAISON D'ADAPALENE ET DE PEROXYDE DE BENZOLE DANS LE TRAITEMENT DE L'ACNE
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 43502
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 09542779
Estimated Expiration: ⤷ Get Started Free
Patent: 14040481
Patent: COMBINATIONS OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 16029094
Patent: 座瘡病変の治療のためのアダパレンと過酸化ベンゾイルとの組合せ (COMBINATIONS OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09000319
Patent: COMBINACION DE ADAPALENO Y PEROXIDO DE BENZOILO PARA TRATAMIENTO DE LESIONES DEL ACNE. (COMBINATION OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS.)
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 46318
Estimated Expiration: ⤷ Get Started Free
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 46318
Estimated Expiration: ⤷ Get Started Free
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 09104949
Patent: КОМБИНАЦИЯ АДАПАЛЕНА И ПЕРОКСИДА БЕНЗОИЛА ДЛЯ ЛЕЧЕНИЯ ПОРАЖЕНИЙ АКНЕ
Estimated Expiration: ⤷ Get Started Free
Patent: 12136952
Patent: КОМБИНАЦИЯ АДАПАЛЕНА И ПЕРОКСИДА БЕНЗОИЛА ДЛЯ ЛЕЧЕНИЯ ПОРАЖЕНИЙ АКНЕ
Estimated Expiration: ⤷ Get Started Free
Patent: 12144414
Patent: КОМБИНАЦИЯ АДАПАЛЕНА И ПЕРОКСИДА БЕНЗОИЛА ДЛЯ ЛЕЧЕНИЯ ПОРАЖЕНИЙ АКНЕ
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 46318
Estimated Expiration: ⤷ Get Started Free
Patent: 50035
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 090028764
Patent: COMBINATION OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 150003917
Patent: COMBINATION OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 160120352
Patent: 여드름 병변 치료를 위한 아다팔렌 및 벤조일 퍼옥시드의 조합물 (COMBINATION OF ADAPALENE AND BENZOYL PEROXIDE FOR TREATING ACNE LESIONS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 23951
Estimated Expiration: ⤷ Get Started Free
Patent: 03505
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1819658
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EPIDUO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 1266646 | DERIVES BENZONAPHTALENIQUES, LEUR PROCEDE DE PREPARATION ET LEUR APPLICATION DANS LES DOMAINES PHARMACEUTIQUE ET COSMETIQUE (BENZONAPHTALENIC DERIVATIVES, PROCESS FOR THEIR PREPARATION AND USES AS PHARMACEUTIC AND COSMETIC AGENTS) | ⤷ Get Started Free |
| Canada | 2656456 | ⤷ Get Started Free | |
| Japan | 4889922 | ⤷ Get Started Free | |
| Cyprus | 1116672 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EPIDUO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1458369 | 380 | Finland | ⤷ Get Started Free | |
| 1458369 | CA 2008 00029 | Denmark | ⤷ Get Started Free | PRODUCT NAME: ADAPALEN, BENZOYLPEROXID |
| 1458369 | 08C0024 | France | ⤷ Get Started Free | PRODUCT NAME: ADAPALENE - PEROXYDE DE BENZOLE; REGISTRATION NO/DATE IN FRANCE: NL 33724 DU 20080123; REGISTRATION NO/DATE AT EEC: 40440 DU 20071218 |
| 0199636 | 300209 | Netherlands | ⤷ Get Started Free | 300209, 20060411, EXPIRES: 20070702 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for EPIDUO: An In-Depth Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
