EPCLUSA Drug Patent Profile
✉ Email this page to a colleague
When do Epclusa patents expire, and what generic alternatives are available?
Epclusa is a drug marketed by Gilead Sciences Inc and is included in two NDAs. There are sixteen patents protecting this drug.
This drug has five hundred and nineteen patent family members in forty-eight countries.
The generic ingredient in EPCLUSA is sofosbuvir; velpatasvir. There are nine drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the sofosbuvir; velpatasvir profile page.
DrugPatentWatch® Generic Entry Outlook for Epclusa
Epclusa was eligible for patent challenges on June 28, 2020.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 30, 2034. This may change due to patent challenges or generic licensing.
There have been three patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for EPCLUSA?
- What are the global sales for EPCLUSA?
- What is Average Wholesale Price for EPCLUSA?
Summary for EPCLUSA
| International Patents: | 519 |
| US Patents: | 16 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 40 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for EPCLUSA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EPCLUSA |
| What excipients (inactive ingredients) are in EPCLUSA? | EPCLUSA excipients list |
| DailyMed Link: | EPCLUSA at DailyMed |
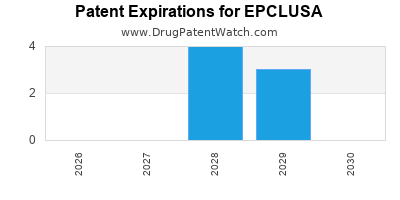
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for EPCLUSA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for EPCLUSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Peking University People's Hospital | Phase 4 |
| Macfarlane Burnet Institute for Medical Research and Public Health Ltd | Phase 4 |
| The University of Queensland | Phase 4 |
Pharmacology for EPCLUSA
US Patents and Regulatory Information for EPCLUSA
EPCLUSA is protected by sixteen US patents and seven FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of EPCLUSA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting EPCLUSA
Combination formulation of two antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Combination formulation of two antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidate prodrugs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidate prodrugs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidate prodrugs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidates
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidates
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidate prodrugs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Compositions and methods for treating hepatitis C virus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidate prodrugs
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Nucleoside phosphoramidates
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Combination formulation of two antiviral compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting EPCLUSA
FOR TREATMENT OF PEDIATRIC PATIENTS 3 YEARS OF AGE TO LESS THAN 6 YEARS OF AGE WEIGHING LESS THAN 17 KG WITH CHRONIC HEPATITIS C VIRUS (HCV) GENOTYPE 1, 2, 3, 4, 5, OR 6 INFECTION: WITHOUT CIRRHOSIS OR WITH COMPENSATED CIRRHOSIS; OR WITH DECOMPENSATED CIRRHOSIS FOR USE IN COMBINATION WITH RIBAVIRIN
Exclusivity Expiration: ⤷ Sign Up
UPDATES THE US PRESCRIBING INFORMATION WITH CLINICAL DATA REGARDING THE USE OF SOFOSBUVIR AND VELPATASVIR FOR THE TREATMENT OF CHRONIC HCV GENOTYPE 1, 2, 3, 4, 5, OR 6 INFECTION IN PEOPLE WHO INJECT DRUGS (PWID), INCLUDING THOSE ON MEDICATION-ASSISTED TREATMENT (MAT) FOR OPIOID USE DISORDER
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
TX OF PED PTS 6 YRS OF AGE & OLDER OR WEIGHING AT LEAST 17 KG WITH CHRONIC HEPATITIS C VIRUS GENOTYPE 1, 2, 3, 4, 5, OR 6 INFECTION: WITHOUT CIRRHOSIS OR WITH COMPENSATED CIRRHOSIS; OR WITH DECOMPENSATED CIRRHOSIS FOR USE IN COMBINATION WITH RIBAVIRIN
Exclusivity Expiration: ⤷ Sign Up
INFORMATION ADDED TO THE LABELING DESCRIBING A PHASE 2, MULTICENTER, OPEN-LABEL STUDY TO EVALUATE THE SAFETY/EFFICACY OF SOFOSBUVIR/VELPATASVIR IN SUBJECTS WITH CHRONIC HCV INFECTION WHO HAVE RECEIVED A LIVER TRANSPLANT
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | EPCLUSA | sofosbuvir; velpatasvir | TABLET;ORAL | 208341-001 | Jun 28, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Gilead Sciences Inc | EPCLUSA | sofosbuvir; velpatasvir | TABLET;ORAL | 208341-002 | Mar 19, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Gilead Sciences Inc | EPCLUSA | sofosbuvir; velpatasvir | PELLETS;ORAL | 214187-002 | Jun 10, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for EPCLUSA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Ireland UC | Epclusa | sofosbuvir, velpatasvir | EMEA/H/C/004210 Epclusa is indicated for the treatment of chronic hepatitis C virus (HCV) infection in patients 3 years of age and older (see sections 4.2, 4.4 and 5.1). |
Authorised | no | no | no | 2016-07-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for EPCLUSA
When does loss-of-exclusivity occur for EPCLUSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5133
Patent: FORMULACIÓN DE COMBINACIÓN DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 14311827
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Patent: 17276223
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Patent: 19264624
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 21160
Patent: PREPARATION COMBINEE DE DEUX COMPOSES ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
China
Patent: 5517540
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1690473
Patent: КОМБИНИРОВАННЫЙ СОСТАВ ДВУХ ПРОТИВОВИРУСНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 38601
Patent: PRÉPARATION COMBINÉE DE DEUX COMPOSÉS ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Patent: 50014
Patent: FORMULATION DE COMBINAISON DE DEUX COMPOSÉS ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Patent: 05560
Patent: FORMULATION DE COMBINAISON DE DEUX COMPOSÉS ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 20392
Patent: 兩種抗病毒化合物的複方製劑 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Patent: 25626
Patent: 兩種抗病毒化合物的複方製劑 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 60607
Estimated Expiration: ⤷ Sign Up
Patent: 16529293
Patent: 2つの抗ウイルス化合物の組合せ製剤
Estimated Expiration: ⤷ Sign Up
Patent: 17222718
Patent: 2つの抗ウイルス化合物の組合せ製剤 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 16002185
Patent: FORMULACION COMBINADA DE DOS COMPUESTOS ANTIVIRALES. (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 6840
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 38601
Estimated Expiration: ⤷ Sign Up
Patent: 50014
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 38601
Estimated Expiration: ⤷ Sign Up
Patent: 50014
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201600919U
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 38601
Estimated Expiration: ⤷ Sign Up
Patent: 50014
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2239196
Estimated Expiration: ⤷ Sign Up
Patent: 160047522
Patent: 2종의 항바이러스 화합물의 조합 제제 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 92503
Estimated Expiration: ⤷ Sign Up
Patent: 00570
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 26048
Estimated Expiration: ⤷ Sign Up
Patent: 1511756
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 300
Patent: FORMULACIÓN DE COMBINACIÓN DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EPCLUSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 5872539 | ⤷ Sign Up | |
| Moldova, Republic of | 4589 | Compoziţie farmaceutică cu conţinut de sofosbuvir şi utilizarea acesteia în tratamentul hepatitei virale C (Pharmaceutical composition comprising sofosbuvir and uses thereof for treating hepatitis C virus) | ⤷ Sign Up |
| Singapore | 176197 | N- [ (2 ' R) -2 ' -DEOXY-2 ' -FLUORO-2 ' -METHYL-P-PHENYL-5 ' -URIDYLYL] -L-ALANINE 1-METHYLETHYL ESTER AND PROCESS FOR ITS PRODUCTION | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EPCLUSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2203462 | 67/2014 | Austria | ⤷ Sign Up | PRODUCT NAME: SOFOSBUVIR; REGISTRATION NO/DATE: EU/1/13/894 20140117 |
| 2203462 | 300704 | Netherlands | ⤷ Sign Up | PRODUCT NAME: SOFOSBUVIR; REGISTRATION NO/DATE: EU/1/13/894/001-002 20140117 |
| 2635588 | C02635588/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: VELPATASVIR + SOFOSBUVIR + VOXILAPREVIR; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66510 08.12.2017 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


