ENVARSUS XR Drug Patent Profile
✉ Email this page to a colleague
When do Envarsus Xr patents expire, and what generic alternatives are available?
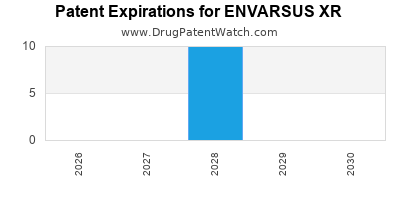
Envarsus Xr is a drug marketed by Veloxis Pharms Inc and is included in one NDA. There are twenty-two patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-six patent family members in twenty-four countries.
The generic ingredient in ENVARSUS XR is tacrolimus. There are twenty drug master file entries for this compound. Thirty-five suppliers are listed for this compound. Additional details are available on the tacrolimus profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Envarsus Xr
A generic version of ENVARSUS XR was approved as tacrolimus by SANDOZ on August 10th, 2009.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for ENVARSUS XR?
- What are the global sales for ENVARSUS XR?
- What is Average Wholesale Price for ENVARSUS XR?
Summary for ENVARSUS XR
| International Patents: | 66 |
| US Patents: | 22 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 80 |
| Clinical Trials: | 49 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for ENVARSUS XR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ENVARSUS XR |
| DailyMed Link: | ENVARSUS XR at DailyMed |

Recent Clinical Trials for ENVARSUS XR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Hospital, Essen | Phase 3 |
| University Health Network, Toronto | Phase 4 |
| University of Alberta | Phase 4 |
Pharmacology for ENVARSUS XR
| Drug Class | Calcineurin Inhibitor Immunosuppressant |
| Mechanism of Action | Calcineurin Inhibitors |
Paragraph IV (Patent) Challenges for ENVARSUS XR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ENVARSUS XR | Extended-release Tablets | tacrolimus | 0.75 mg, 1 mg and 4 mg | 206406 | 1 | 2022-03-31 |
US Patents and Regulatory Information for ENVARSUS XR
ENVARSUS XR is protected by twenty-eight US patents.
Patents protecting ENVARSUS XR
Stabilized tacrolimus composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid dispersions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN PATIENTS CONVERTED FROM TACROLIMUS IMMEDIATE-RELEASE FORMULATIONS
Solid dispersions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN DE NOVO TRANSPLANT PATIENT
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN DE NOVO TRANSPLANT PATIENT
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN PATIENTS CONVERTED FROM TACROLIMUS IMMEDIATE-RELEASE FORMULATIONS
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN DE NOVO TRANSPLANT PATIENT
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN PATIENTS CONVERTED FROM TACROLIMUS IMMEDIATE-RELEASE FORMULATIONS
Stabilized tacrolimus composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid dispersions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid dispersions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN PATIENTS CONVERTED FROM TACROLIMUS IMMEDIATE-RELEASE FORMULATIONS
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN DE NOVO TRANSPLANT PATIENT
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN DE NOVO TRANSPLANT PATIENT
Tacrolimus for improved treatment of transplant patients
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION IN PATIENTS CONVERTED FROM TACROLIMUS IMMEDIATE-RELEASE FORMULATIONS
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PROPHYLAXIS OF ORGAN REJECTION
Stabilized tacrolimus composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modified release compositions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid dispersions comprising tacrolimus
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-003 | Jul 10, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Veloxis Pharms Inc | ENVARSUS XR | tacrolimus | TABLET, EXTENDED RELEASE;ORAL | 206406-002 | Jul 10, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ENVARSUS XR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Astellas Pharma Europe B.V. | Modigraf | tacrolimus | EMEA/H/C/000954 Prophylaxis of transplant rejection in adult and paediatric, kidney, liver or heart allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult and paediatric patients. |
Authorised | no | no | no | 2009-05-15 | |
| Astellas Pharma Europe BV | Advagraf | tacrolimus | EMEA/H/C/000712 Prophylaxis of transplant rejection in adult kidney or liver allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. |
Authorised | no | no | no | 2007-04-23 | |
| Chiesi Farmaceutici S.p.A. | Envarsus | tacrolimus | EMEA/H/C/002655 Prophylaxis of transplant rejection in adult kidney or liver allograft recipients. Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. |
Authorised | no | no | no | 2014-07-18 | |
| LEO Pharma A/S | Protopic | tacrolimus | EMEA/H/C/000374 Flare treatmentAdults and adolescents (16 years of age and above)Treatment of moderate to severe atopic dermatitis in adults who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids.Children (two years of age and above)Treatment of moderate to severe atopic dermatitis in children (two years of age and above) who failed to respond adequately to conventional therapies such as topical corticosteroids.Maintenance treatmentMaintenance treatment of moderate to severe atopic dermatitis for the prevention of flares and the prolongation of flare-free intervals in patients experiencing a high frequency of disease exacerbations (i.e. occurring four or more times per year) who have had an initial response to a maximum of six weeks treatment of twice daily tacrolimus ointment (lesions cleared, almost cleared or mildly affected). |
Authorised | no | no | no | 2002-02-27 | |
| Teva B.V. | Tacforius | tacrolimus | EMEA/H/C/004435 Prophylaxis of transplant rejection in adult kidney or liver allograft recipients.Treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. |
Authorised | yes | no | no | 2017-12-08 | |
| Astellas Pharma GmbH | Protopy | tacrolimus | EMEA/H/C/000375 Treatment of moderate to severe atopic dermatitis in adults who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids. Treatment of moderate to severe atopic dermatitis in children (2 years of age and above) who failed to respond adequately to conventional therapies such as topical corticosteroids.Maintenance treatment of moderate to severe atopic dermatitis for the prevention of flares and the prolongation of flare-free intervals in patients experiencing a high frequency of disease exacerbations (i.e. occurring 4 or more times per year) who have had an initial response to a maximum of 6 weeks treatment of twice daily tacrolimus ointment (lesions cleared, almost cleared or mildly affected). |
Withdrawn | no | no | no | 2002-02-28 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ENVARSUS XR
See the table below for patents covering ENVARSUS XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 1663217 | ⤷ Sign Up | |
| European Patent Office | 2167033 | FORME PHARMACEUTIQUE ORALE À ADMINISTRER UNE FOIS PAR JOUR COMPORTANT DU TACROLIMUS (ONCE DAILY ORAL DOSAGE FORM COMPRISING TACROLIMUS) | ⤷ Sign Up |
| Norway | 334986 | ⤷ Sign Up | |
| Slovenia | 1663216 | ⤷ Sign Up | |
| China | 101869561 | Tacrolimus-containing improved release composition | ⤷ Sign Up |
| World Intellectual Property Organization (WIPO) | 2008106894 | ⤷ Sign Up | |
| World Intellectual Property Organization (WIPO) | 2010005980 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


