EMTRIVA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Emtriva, and what generic alternatives are available?
Emtriva is a drug marketed by Gilead and is included in two NDAs.
The generic ingredient in EMTRIVA is emtricitabine. There are eighteen drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the emtricitabine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Emtriva
A generic version of EMTRIVA was approved as emtricitabine by CIPLA on July 2nd, 2018.
Summary for EMTRIVA
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 139 |
| Clinical Trials: | 13 |
| Patent Applications: | 5,194 |
| Formulation / Manufacturing: | see details |
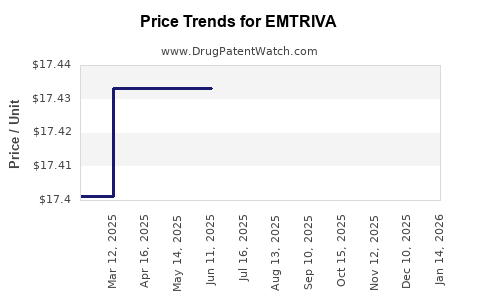
| Drug Prices: | Drug price information for EMTRIVA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EMTRIVA |
| What excipients (inactive ingredients) are in EMTRIVA? | EMTRIVA excipients list |
| DailyMed Link: | EMTRIVA at DailyMed |
Paragraph IV (Patent) Challenges for EMTRIVA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EMTRIVA | Capsules | emtricitabine | 200 mg | 021500 | 1 | 2012-07-16 |
US Patents and Regulatory Information for EMTRIVA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead | EMTRIVA | emtricitabine | CAPSULE;ORAL | 021500-001 | Jul 2, 2003 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Gilead | EMTRIVA | emtricitabine | SOLUTION;ORAL | 021896-001 | Sep 28, 2005 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EMTRIVA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead | EMTRIVA | emtricitabine | SOLUTION;ORAL | 021896-001 | Sep 28, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Gilead | EMTRIVA | emtricitabine | SOLUTION;ORAL | 021896-001 | Sep 28, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Gilead | EMTRIVA | emtricitabine | CAPSULE;ORAL | 021500-001 | Jul 2, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Gilead | EMTRIVA | emtricitabine | CAPSULE;ORAL | 021500-001 | Jul 2, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Gilead | EMTRIVA | emtricitabine | CAPSULE;ORAL | 021500-001 | Jul 2, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Gilead | EMTRIVA | emtricitabine | CAPSULE;ORAL | 021500-001 | Jul 2, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for EMTRIVA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Ireland UC | Emtriva | emtricitabine | EMEA/H/C/000533 Emtriva is indicated for the treatment of HIV-1 infected adults and children in combination with other antiretroviral agents.This indication is based on studies in treatment-naive patients and treatment-experienced patients with stable virological control. There is no experience of the use of Emtriva in patients who are failing their current regimen or who have failed multiple regimens.When deciding on a new regimen for patients who have failed an antiretroviral regimen, careful consideration should be given to the patterns of mutations associated with different medicinal products and the treatment history of the individual patient. Where available, resistance testing may be appropriate. |
Authorised | no | no | no | 2003-10-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for EMTRIVA
See the table below for patents covering EMTRIVA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2513440 | ACTIVITE ET RESOLUTION ANTIVIRALE DU 2-HYDROXYMETHYL-5-(5-FLUOROCYTOSINE-1-YL)-1,3-OXATHIOLANE (ANTIVIRAL ACTIVITY AND RESOLUTION OF 2-HYDROXYMETHYL-5-(5-FLUOROCYTOSIN-1-YL)-1,3-OXATHIOLANE) | ⤷ Sign Up |
| Russian Federation | 2116789 | METHOD OF INHIBITION OF HEPATITIS B VIRAL INFECTIONS | ⤷ Sign Up |
| Ireland | 20060148 | ⤷ Sign Up | |
| Norway | 2008010 | ⤷ Sign Up | |
| European Patent Office | 1808434 | ⤷ Sign Up | |
| Ireland | 920701 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EMTRIVA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3808743 | CA 2022 00035 | Denmark | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF RILPIVIRINE OR A THERAPEUTICALLY EQUIVALENT FORM THEREOF PROTECTED BY THE BASIC PATENT, SUCH AS A PHARMACEUTICALLY ACCEPTABLE ADDITION SALT OF RILPIVIRINE, INCLUDING THE HYDROCHLORIC ACID SALT OF RILPIVIRINE, AND EMTRICITABINE; REG. NO/DATE: EU/1/11/737/001-002 20111128 |
| 1663240 | 92853 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COMBINAISON DE RILPIVIRINE OU UNE FORME THERAPEUTIQUE EQUIVALENTE QUI EN DERIVE TELLE QUE PROTEGEE PAR LE BREVET DE BASE, TEL QU'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE RILPIVIRINE, INCLUANT LE SEL CHLORHYDRATE DE RILPIVIRINE ET L'EMTRICITABINE |
| 0513200 | C00513200/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: EMTRICITABINE; REGISTRATION NUMBER/DATE: SWISSMEDIC 56880 25.10.2004 |
| 1663240 | 1690062-3 | Sweden | ⤷ Sign Up | PRODUCT NAME: A COMBINATION OF: RILPIVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT OF RILPIVIRINE, INCLUDING THE HYDROCHLORIDE SALT OF RILPIVIRINE; EMTRICITABINE; AND TENOFOVIR ALAFENAMIDE, OR A PHARMCEUTICALLY ACCEPTABLE SALT THEREOF, INCLUDING TENOFOVIR ALAFENAMIDE FUMARATE.; REG. NO/DATE: EU/1/16/1112 20160623 |
| 0513200 | SPC/GB04/016 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: EMTRICITABINE OR SALTS AND ESTERS THEREOF; REGISTERED: UK EU/1/03/261/001 20031024; UK EU/1/03/261/002 20031024; UK EU/1/03/261/003 20031024 |
| 1663240 | 300851 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN: - RILPIVIRINE OF EEN THERAPEUTISCH EQUIVALENTE VORM DAARVAN ZOALS BESCHERMD DOOR HET BASISOCTROOI, ZOALS EEN FARMACEUTISCH AANVAARDBAAR ZOUT VAN RILPIVIRINE, WAARONDER HET HYDROCHLORIDEZOUT VAN RILPIVIRINE; - EMTRICITABINE; EN - TENOFOVIRALAFENAMIDE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, IN HET BIJZONDER TENOFOVIRALAFENAMIDEFUMARAAT; REGISTRATION NO/DATE: EU/1/16/1112 20160623 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


