ELLA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ella, and what generic alternatives are available?
Ella is a drug marketed by Lab Hra Pharma and is included in one NDA. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-five patent family members in twenty-eight countries.
The generic ingredient in ELLA is ulipristal acetate. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the ulipristal acetate profile page.
DrugPatentWatch® Generic Entry Outlook for Ella
Ella was eligible for patent challenges on August 13, 2014.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ELLA?
- What are the global sales for ELLA?
- What is Average Wholesale Price for ELLA?
Summary for ELLA
| International Patents: | 65 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 70 |
| Patent Applications: | 1,041 |
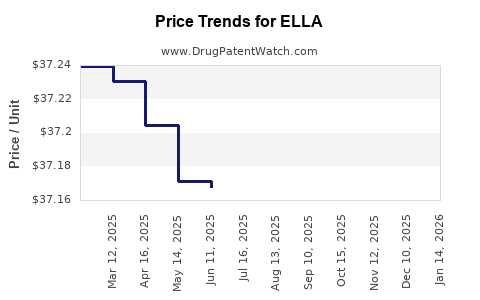
| Drug Prices: | Drug price information for ELLA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ELLA |
| What excipients (inactive ingredients) are in ELLA? | ELLA excipients list |
| DailyMed Link: | ELLA at DailyMed |
Pharmacology for ELLA
| Drug Class | Progesterone Agonist/Antagonist |
| Mechanism of Action | Selective Progesterone Receptor Modulators |
Paragraph IV (Patent) Challenges for ELLA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ELLA | Tablets | ulipristal acetate | 30 mg | 022474 | 1 | 2014-08-13 |
US Patents and Regulatory Information for ELLA
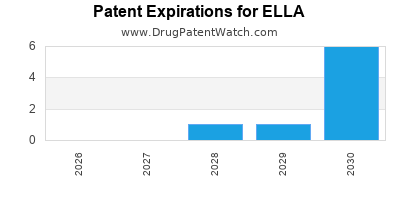
ELLA is protected by eight US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 8,426,392 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 9,844,510 | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 10,159,681 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ELLA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Laboratoire HRA Pharma | ellaOne | ulipristal acetate | EMEA/H/C/001027Emergency contraception within 120 hours (five days) of unprotected sexual intercourse or contraceptive failure. | Authorised | no | no | no | 2009-05-15 | |
| Gedeon Richter Plc. | Ulipristal Acetate Gedeon Richter | ulipristal acetate | EMEA/H/C/005017Ulipristal acetate is indicated for one treatment course of pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age.Ulipristal acetate is indicated for intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age who are not eligible for surgery. | Withdrawn | no | no | no | 2018-08-27 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ELLA
When does loss-of-exclusivity occur for ELLA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09326084
Patent: Ulipristal acetate tablets
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0922796
Patent: COMPRIMIDO FARMACÊUTICO PARA ADMINISTRAÇÃO POR VIA ORAL E MÉTODO DE FABRICAÇÃO DO MESMO
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 45084
Patent: COMPRIMES D'ULIPRISTAL ACETATE (ULIPRISTAL ACETATE TABLETS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2245173
Patent: Ulipristal acetate tablets
Estimated Expiration: ⤷ Get Started Free
Patent: 5267168
Patent: Ulipristal Acetate Tablets
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 00186
Patent: TABLETAS DE ACETATO DE ULIPRISTAL
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0161262
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18099
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 65800
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 65800
Patent: COMPRIMÉS D'ULIPRISTAL ACÉTATE (ULIPRISTAL ACETATE TABLETS)
Estimated Expiration: ⤷ Get Started Free
Patent: 03445
Patent: COMPRIMÉS D'ACÉTATE D'ULIPRISTAL (ULIPRISTAL ACETATE TABLETS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 32134
Patent: 烏利司他醋酸片 (ULIPRISTAL ACETATE TABLETS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 30762
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3247
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 84502
Estimated Expiration: ⤷ Get Started Free
Patent: 51727
Estimated Expiration: ⤷ Get Started Free
Patent: 12511041
Estimated Expiration: ⤷ Get Started Free
Patent: 15107994
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 65800
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 3358
Estimated Expiration: ⤷ Get Started Free
Patent: 11006106
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3498
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 65800
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 65800
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 92853
Estimated Expiration: ⤷ Get Started Free
Patent: 11127989
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01600372
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 209
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 65800
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1104137
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1733533
Estimated Expiration: ⤷ Get Started Free
Patent: 110097936
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 96554
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1863
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ELLA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Cyprus | 1118099 | ⤷ Get Started Free | |
| Australia | 2010237120 | ⤷ Get Started Free | |
| Russian Federation | 2011146058 | СПОСОБ КОНТРАЦЕПЦИИ, ИСПОЛЬЗУЕМЫЙ ПО МЕРЕ НЕОБХОДИМОСТИ | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: ELLA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
