Last updated: July 30, 2025
Introduction
In the dynamic and highly competitive pharmaceutical industry, understanding a company's market position, strengths, and strategic trajectory is vital for stakeholders, investors, and partners. Lab Hra Pharma, a notable entity within this sector, exemplifies unique competitive advantages and challenges shaped by its innovation pipeline, strategic alliances, and market penetration strategies. This analysis deciphers Lab Hra Pharma's position within the broader pharmaceutical landscape, highlighting its core strengths and offering targeted strategic insights to inform future growth opportunities.
Market Position of Lab Hra Pharma
Global and Regional Footprint
Lab Hra Pharma has established a significant footprint across key markets, including North America, Europe, and Asia-Pacific. While not yet a market leader like Pfizer or Novartis, the company’s nimbleness and focus on niche therapeutic areas have enabled steady growth. Its regional presence is primarily driven by a robust distribution network and localized R&D centers, facilitating tailored solutions that cater to regional health needs.
Segment Focus and Product Portfolio
Lab Hra Pharma specializes in oncology, rare diseases, and metabolic disorders, sectors characterized by high unmet medical needs and sustained pharmaceutical investment. Its portfolio includes a mix of proprietary compounds, biosimilars, and combination therapies, positioning itself as an innovative yet cost-effective alternative in competitive segments. The company’s pipelines show promising developments in immunotherapies and gene therapies, aligning with industry trends toward personalized medicine.
Market Share and Competitive Rank
While precise market share figures remain proprietary, industry estimates place Lab Hra Pharma among mid-tier players with a notable presence in specific therapeutic niches. Its growth rate surpasses many traditional mid-size competitors, driven by an emphasis on innovation and strategic acquisitions. As patent cliffs loom for larger firms, Lab Hra Pharma's agility and focus on R&D are poised to capitalize on emerging opportunities.
Core Strengths of Lab Hra Pharma
Robust R&D Pipeline
Lab Hra Pharma invests approximately 20% of its revenue into R&D, facilitating a pipeline rich in investigational drugs with high therapeutic potential. The company's focus on precision medicine and biologics aligns it with future industry directions, enabling early-stage differentiation and the prospect of sustainable revenue streams from novel therapies.
Strategic Collaborations and Partnerships
The company's strategic alliances with biotech firms, academic institutions, and contract manufacturing organizations (CMOs) amplify its innovation capacity and reduce developmental costs. These collaborations accelerate go-to-market timelines and facilitate access to cutting-edge technologies, such as antibody-drug conjugates (ADCs) and gene editing platforms.
Regulatory and Market Access Expertise
Lab Hra Pharma has cultivated expertise navigating complex regulatory landscapes across multiple jurisdictions. Its proactive engagement with health authorities expedites approval processes and ensures compliance, offering a competitive advantage in launching new therapies swiftly.
Operational Efficiency and Cost Management
Through streamlined manufacturing processes and supply chain management, Lab Hra Pharma maintains competitive pricing strategies. Its lean operational model enables rapid scaling in response to demand surges, minimizing costs associated with R&D failure and manufacturing delays.
Strategic Insights for Future Growth
Innovation Focus and Pipeline Expansion
To sustain competitive differentiation, Lab Hra Pharma should prioritize expanding its pipeline into newer modalities, including cell therapies and mRNA-based treatments. Investment in diagnostics and companion diagnostics can augment its precision medicine offerings, enhancing value propositions for payers and providers.
Geographic Expansion
Expanding into emerging markets with tailored, affordable therapies could significantly boost revenue. Establishing local manufacturing and distribution hubs in regions like Southeast Asia and Latin America will improve market penetration and affirm a local presence, which is increasingly critical for global acceptance.
Digital Transformation and Data Utilization
Leveraging big data analytics, AI-driven drug discovery, and digital clinical trial platforms can accelerate development timelines and reduce costs. Digital engagement with healthcare providers and patients promotes better adherence and real-world evidence collection, informing ongoing product refinement.
Enhancing Patent Portfolio and Intellectual Property Strategy
Strengthening patent protection, especially in high-growth areas like biologics and gene therapies, safeguards competitive advantage. A proactive IP strategy encompassing collaborations for patent filing and licensing can optimize revenue potential from innovations.
Sustainable and Accessible Therapies
Aligning with the global push towards sustainable healthcare, Lab Hra Pharma should develop affordable therapies that meet unmet needs in low- and middle-income countries. Incorporating environmentally sustainable practices into manufacturing and R&D operations will bolster corporate responsibility credentials.
Competitive Challenges and Risks
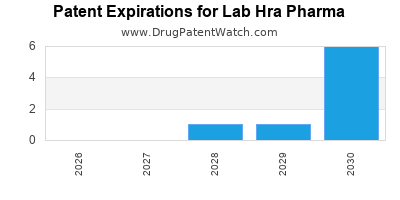
Despite its strengths, Lab Hra Pharma faces several risks, including patent expirations, intense industry competition, and regulatory uncertainties. The rapid pace of technological change necessitates continuous innovation and agility, while geopolitical and economic factors can impact market access and supply chains.
Conclusion
Lab Hra Pharma’s strategic positioning around niche therapeutic segments, combined with its R&D prowess and collaborative approach, underscores its potential as a formidable player in the pharmaceutical landscape. To capitalize on emerging opportunities, the company must maintain agility, deepen its innovation capabilities, and expand its geographic and therapeutic footprint. Strategic investments in digital transformation and sustainable development will further enhance its market competitiveness and stakeholder value.
Key Takeaways
- Lab Hra Pharma’s niche focus in oncology and rare diseases positions it well amidst industry shifts toward personalized medicine; continuous pipeline innovation remains critical.
- Strategic alliances and regulatory expertise provide a competitive edge, enabling faster market access and enhanced R&D efficiency.
- Geographic expansion into emerging markets offers growth opportunities, provided tailored, affordable therapies are prioritized.
- Digital transformation, including AI and big data, can significantly accelerate drug development and optimize patient engagement.
- Strengthening patent portfolios and emphasizing sustainable practices will underpin long-term competitiveness and social responsibility.
Frequently Asked Questions (FAQs)
1. How does Lab Hra Pharma differentiate itself from major pharmaceutical giants?
Lab Hra Pharma focuses on niche therapeutic areas like oncology and rare diseases, investing heavily in innovative biologics and precision medicine. Its agility, strategic collaborations, and tailored regional approaches enable it to compete effectively despite lacking the extensive infrastructure of industry leaders.
2. What are the primary growth opportunities for Lab Hra Pharma?
Key opportunities include expanding its pipeline into advanced modalities like gene and cell therapies, entering emerging markets with affordable products, and leveraging digital tools for faster drug development and delivery.
3. How does Lab Hra Pharma manage regulatory risks across different markets?
The company maintains a dedicated regulatory affairs team with local expertise, proactive engagement with health authorities, and adaptive clinical strategies to streamline approvals and ensure compliance.
4. What challenges does Lab Hra Pharma face in sustaining growth?
Major challenges include patent expirations of mature products, competitive pressure from both generic and innovative firms, evolving regulatory landscapes, and geopolitical uncertainties that could affect market access.
5. What role does digital innovation play in Lab Hra Pharma’s strategic plan?
Digital innovation, including AI-driven drug discovery, digital clinical trials, and data analytics, is central to reducing development timelines, lowering costs, and enhancing patient engagement—key factors in maintaining competitive advantage.
Sources:
[1] Industry reports on pharmaceutical market segmentation and company strategies.
[2] Lab Hra Pharma corporate disclosures and R&D investment data.
[3] Market analysis articles from sector-leading publications.