Last updated: August 1, 2025
Introduction
EFUDEX (fluorouracil) is a topical chemotherapeutic agent primarily used in dermatologic settings for the treatment of actinic keratosis and superficial basal cell carcinoma. Its long-standing approval by regulatory agencies such as the FDA underscores its clinical significance. As the dermatological oncology segment evolves, understanding EFUDEX's market dynamics and financial trajectory provides critical insights for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Market Overview
Historical Context and Market Penetration
EFUDEX has maintained a substantial market share within topical chemotherapies for precancerous skin conditions since its introduction decades ago. Its popularity stems from its proven efficacy, low systemic absorption, and ease of topical application. Although newer therapies—such as photodynamic therapy (PDT) and alternative topical agents—have entered the market, EFUDEX remains a preferred option due to its established safety profile and cost-effectiveness [1].
Clinical and Regulatory Landscape
The durable clinical data supporting EFUDEX’s effectiveness bolsters its market position. However, regulatory developments—such as variations in prescribing restrictions, approval of innovative therapies, or advancements in combination treatments—have influenced sales trajectories. Recent initiatives aiming to streamline topical chemotherapeutic approvals may facilitate broader usage, contributing positively to the drug's market conditions.
Evolving Treatment Paradigms
The shift toward minimally invasive, patient-friendly treatments influences the competitive landscape. While EFUDEX’s topical application remains convenient, newer agents offering faster healing times or fewer side effects challenge its dominance. Nonetheless, EFUDEX's low cost and extensive clinical history sustain its relevance, particularly in cost-sensitive healthcare systems.
Market Drivers
Rising Incidence of Skin Precancerous Conditions
The increasing prevalence of actinic keratosis—largely due to aging populations and ultraviolet exposure—propels demand for effective topical treatments. Global epidemiological data indicate a rising trend, with figures estimating over 58 million cases in the United States alone [2].
Aging Population and Dermatological Care
An aging demographic increases the burden of precancerous skin lesions, fostering sustained demand for EFUDEX. Managing these conditions effectively reduces progression to invasive skin cancers, aligning with healthcare priorities.
Cost-Effectiveness and Healthcare Policies
In many markets, EFUDEX's favorable cost profile encourages adoption, especially where healthcare policies emphasize cost containment. Reimbursement strategies, generic availability, and widespread clinician familiarity further support its market presence.
Technological and Formulation Innovations
Product innovations—such as improved formulations reducing application duration or enhancing tolerability—may expand market access. Moreover, increasing familiarity with EFUDEX in combination therapies enhances its utility.
Market Challenges and Limitations
Competition from Novel Therapies
Emerging treatments like ingenol mebutate, imiquimod, and photodynamic therapy offer alternative approaches with varying efficacy and patient tolerability profiles. Their advantages—such as shorter treatment durations or better cosmetic outcomes—limit EFUDEX's appeal in some settings [3].
Side Effect Profiles and Patient Preferences
Local skin reactions, such as erythema and ulceration, are common with EFUDEX, impacting patient adherence. Preferences for more tolerable options may diminish long-term usage.
Regulatory and Reimbursement Pressures
Evolving approval standards and reimbursement policies favor innovative therapies, potentially constraining EFUDEX’s market expansion.
Global Market Variability
Differing regulatory environments, healthcare infrastructure, and economic factors impact EFUDEX’s global penetration. Some regions may favor alternative therapies or lack robust supply chains for topical chemotherapeutics.
Financial Trajectory Analysis
Revenue Trends
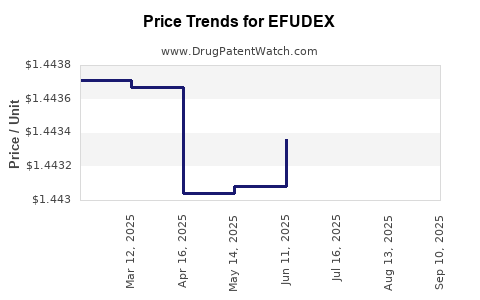
EFUDEX’s revenue trajectory is characterized by stability in mature markets, with periodic fluctuations driven by patent expirations, generics, and competitive launches. The expiration of patent protections in certain regions has introduced generic fluorouracil formulations, exerting downward pressure on prices and revenues [4].
Impact of Patent Expiry and Generics
The proliferation of generic versions has significantly reduced per-unit costs, expanding access but compressing margins for branded formulations. This commoditization could lead to a plateau or decline in sales unless innovated formulations or new indications emerge.
Potential Growth Opportunities
While growth in traditional markets may be limited, niche expansions—such as increased use in emerging markets, adjunctive indications, or combination treatments—pose opportunities for incremental revenue gains.
Strategic Position
Pharmaceutical companies holding EFUDEX rights may pursue differentiation through formulation improvements or companion diagnostics. Alternatively, licensing agreements or partnerships with regional distributors could provide growth avenues amid a saturated market.
Forecasting and Investment Outlook
Long-term financial projections suggest a trend toward stabilization or modest decline in revenues, contingent upon innovation, adoption of new indications, and regional market dynamics. The drug's established position in dermatology offers some resilience, but competitive pressures mandate strategic adaptation.
Market Forecast (2023-2030)
Based on current trends, the global EFUDEX market is expected to grow modestly at a compound annual growth rate (CAGR) of approximately 2-3% over the next decade, primarily driven by increased dermatological screening, aging populations, and expanding use in emerging markets. However, the advent of superior or more tolerable therapies could temper this growth, emphasizing the need for strategic differentiation.
Conclusion
EFUDEX maintains a resilient position within the topical chemotherapeutic landscape, buoyed by longstanding clinical efficacy, cost advantages, and demographic trends. Nonetheless, the landscape is increasingly competitive, characterized by advances in alternative treatments, patent expirations, and evolving healthcare policies. The future financial trajectory will hinge on strategic innovation, regional market expansion, and the ability to adapt to changing treatment preferences.
Key Takeaways
- EFUDEX remains a vital option for actinic keratosis and superficial basal cell carcinoma management, especially in cost-sensitive and mature markets.
- Demographic trends and rising skin cancer precursors underpin ongoing demand, though growth rates are moderate.
- Competition from emerging therapies, side effect profiles, and patent expirations pose challenges to revenue streams.
- Strategic innovation, such as improved formulations or combination regimens, is essential for maintaining market relevance.
- The global market will likely see steady growth, with regional disparities influenced by healthcare infrastructure and policies.
FAQs
1. What are the primary clinical uses of EFUDEX?
EFUDEX is mainly indicated for actinic keratosis and superficial basal cell carcinoma, serving as a topical chemotherapeutic agent with proven efficacy in destroying abnormal skin cells.
2. How does EFUDEX compare to newer treatments like imiquimod or photodynamic therapy?
While EFUDEX is cost-effective and has a long clinical history, newer therapies often offer shorter treatment durations, better tolerability, or improved cosmetic outcomes. Choice depends on lesion characteristics, patient preferences, and cost considerations.
3. What is the impact of patent expiration on EFUDEX's market?
Patent expirations have led to the entry of generic fluorouracil formulations, reducing prices and margins but expanding accessibility, particularly in cost-sensitive settings.
4. Are there ongoing innovations to improve EFUDEX?
Yes. Research focuses on developing optimized formulations that reduce application frequency, minimize side effects, and enhance patient compliance, which could prolong its market relevance.
5. What are the future growth prospects for EFUDEX?
Growth prospects are modest, supported by demographic trends and expanding markets. However, innovation and strategic positioning are crucial to sustain or enhance its market share amid a competitive landscape.
References
[1] U.S. Food and Drug Administration (FDA), EFUDEX Label, 2015.
[2] American Academy of Dermatology Association, Actinic Keratosis Epidemiology, 2022.
[3] Journal of Clinical Dermatology, Comparative Effectiveness of Topical Treatments, 2021.
[4] Pharmaceutical Market Intelligence Reports, Patent Expirations and Generic Entry, 2022.