EDARBYCLOR Drug Patent Profile
✉ Email this page to a colleague
When do Edarbyclor patents expire, and when can generic versions of Edarbyclor launch?
Edarbyclor is a drug marketed by Azurity and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and seventeen patent family members in forty-four countries.
The generic ingredient in EDARBYCLOR is azilsartan kamedoxomil; chlorthalidone. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the azilsartan kamedoxomil; chlorthalidone profile page.
DrugPatentWatch® Generic Entry Outlook for Edarbyclor
Edarbyclor was eligible for patent challenges on February 25, 2015.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EDARBYCLOR?
- What are the global sales for EDARBYCLOR?
- What is Average Wholesale Price for EDARBYCLOR?
Summary for EDARBYCLOR
| International Patents: | 117 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 1 |
| Patent Applications: | 34 |
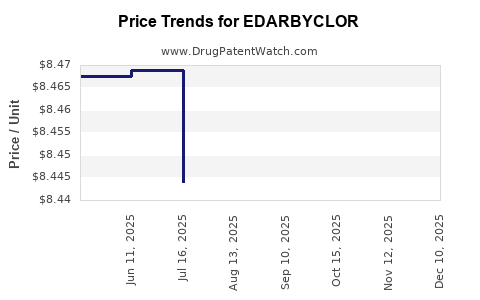
| Drug Prices: | Drug price information for EDARBYCLOR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EDARBYCLOR |
| What excipients (inactive ingredients) are in EDARBYCLOR? | EDARBYCLOR excipients list |
| DailyMed Link: | EDARBYCLOR at DailyMed |
Recent Clinical Trials for EDARBYCLOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Takeda | Phase 3 |
Pharmacology for EDARBYCLOR
| Drug Class | Angiotensin 2 Receptor Blocker Thiazide-like Diuretic |
| Mechanism of Action | Angiotensin 2 Type 1 Receptor Antagonists |
| Physiological Effect | Decreased Blood Pressure Increased Diuresis |
Paragraph IV (Patent) Challenges for EDARBYCLOR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EDARBYCLOR | Tablets | azilsartan kamedoxomil; chlorthalidone | 40 mg/12.5 mg and 40 mg/25 mg | 202331 | 1 | 2022-04-19 |
US Patents and Regulatory Information for EDARBYCLOR
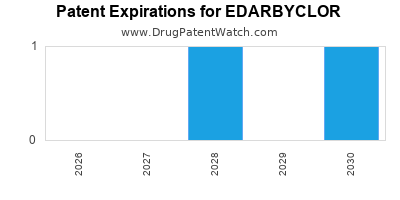
EDARBYCLOR is protected by five US patents.
Expired US Patents for EDARBYCLOR
International Patents for EDARBYCLOR
When does loss-of-exclusivity occur for EDARBYCLOR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2883
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09277455
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0916847
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 32018
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11000187
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2164918
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 41633
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 110111
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 011000032
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 11010856
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1170273
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 10385
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0146062
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 35491
Estimated Expiration: ⤷ Get Started Free
Patent: 11529444
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11001150
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 553
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0948
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 110551
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 10385
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1100871
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 110038145
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 1008915
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 11000045
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 3905
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 017
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EDARBYCLOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2645962 | ⤷ Get Started Free | |
| Austria | E370136 | ⤷ Get Started Free | |
| Poland | 1857457 | ⤷ Get Started Free | |
| Norway | 922495 | ⤷ Get Started Free | |
| New Zealand | 549755 | Benzimidazole derivative and use as AII receptor antagonist | ⤷ Get Started Free |
| Portugal | 2310385 | ⤷ Get Started Free | |
| European Patent Office | 2124903 | COMPOSITION PHARMACEUTIQUE SOLIDE COMPRENANT UN DÉRIVÉ DE BENZIMIDAZOLE-7-CARBOXYLATE ET UN AGENT DE CONTRÔLE DU PH (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EDARBYCLOR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1718641 | 2012/008 | Ireland | ⤷ Get Started Free | PRODUCT NAME: AZILSARTAN MEDOXOMIL AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING THE POTASSIUM SALT; REGISTRATION NO/DATE: EU/1/11/734/001-011 EU/1/11/735/001-011 20111209 |
| 1718641 | CA 2012 00013 | Denmark | ⤷ Get Started Free | |
| 1718641 | C20120004 00052 | Estonia | ⤷ Get Started Free | PRODUCT NAME: EDARBI - ASILSARTAANMEDOKSOMIIL;REG NO/DATE: C(2011) 9280 FINAL 07.12.2011 |
| 1718641 | 436 | Finland | ⤷ Get Started Free | |
| 1718641 | 12C0034 | France | ⤷ Get Started Free | PRODUCT NAME: AZILSARTAN MEDOXOMIL ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/11/735/001 20111207 |
| 1718641 | 91962 | Luxembourg | ⤷ Get Started Free | 91962, EXPIRES: 20261207 |
| 1718641 | C01718641/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: AZILSARTAN MEDOXOMIL; REGISTRATION NO/DATE: SWISSMEDIC 62158 31.08.2012 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for EDARBYCLOR
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
