DSUVIA Drug Patent Profile
✉ Email this page to a colleague
When do Dsuvia patents expire, and what generic alternatives are available?
Dsuvia is a drug marketed by Vertical Pharms and is included in one NDA. There are twenty patents protecting this drug.
This drug has one hundred and five patent family members in twenty countries.
The generic ingredient in DSUVIA is sufentanil citrate. There are nine drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the sufentanil citrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Dsuvia
A generic version of DSUVIA was approved as sufentanil citrate by HIKMA on December 15th, 1995.
Summary for DSUVIA
| International Patents: | 105 |
| US Patents: | 20 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 22 |
| Clinical Trials: | 3 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for DSUVIA |
| What excipients (inactive ingredients) are in DSUVIA? | DSUVIA excipients list |
| DailyMed Link: | DSUVIA at DailyMed |

Recent Clinical Trials for DSUVIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| United States Department of Defense | Phase 3 |
| Frank Guyette | Phase 3 |
| Montefiore Medical Center | Phase 4 |
Pharmacology for DSUVIA
| Drug Class | Opioid Agonist |
| Mechanism of Action | Full Opioid Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for DSUVIA
US Patents and Regulatory Information for DSUVIA
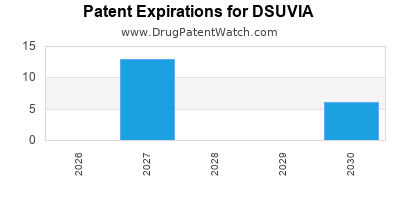
DSUVIA is protected by twenty US patents.
Patents protecting DSUVIA
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small-volume oral transmucosal dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Storage and dispensing devices for administration of oral transmucosal dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Small-volume oral transmucosal dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small-volume oral transmucosal dosage
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Bioadhesive drug formulations for oral transmucosal delivery
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Bioadhesive drug formulations for oral transmucosal delivery
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Storage and dispensing devices for administration of oral transmucosal dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small-volume oral transmucosal dosage forms
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Bioadhesive drug formulations for oral transmucosal delivery
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Sufentanil solid dosage forms comprising oxygen scavengers and methods of using the same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ACUTE PAIN
Small volume oral transmucosal dosage forms containing sufentanil for treatment of pain
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Vertical Pharms | DSUVIA | sufentanil citrate | TABLET;SUBLINGUAL | 209128-001 | Nov 2, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for DSUVIA
See the table below for patents covering DSUVIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2808005 | Dispositifs de stockage et de distribution destinés à l'administration de formes pharmaceutiques transmucosiques orales (Storage and dispensing devices for administration of oral transmucosal dosage forms) | ⤷ Sign Up |
| European Patent Office | 2099406 | Dispositifs de stockage et de distribution destinés à l'administration de formes pharmaceutiques transmucosiques orales (Storage and dispensing devices for administration of oral transmucosal dosage forms) | ⤷ Sign Up |
| South Korea | 101545754 | ⤷ Sign Up | |
| China | 103585021 | Drug storage and dispensing device and system comprising the same | ⤷ Sign Up |
| Australia | 2009316874 | Sufentanil solid dosage forms comprising oxygen scavengers and methods of using the same | ⤷ Sign Up |
| China | 113143769 | 用于分配口腔经粘膜剂型的设备和方法 (APPARATUS AND METHODS FOR DISPENSING ORAL TRANSMUCOSAL DOSAGE FORMS) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DSUVIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2114383 | 122016000023 | Germany | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL, WAHLWEISE IN FORM VON SUFENTANILCITRAT; REGISTRATION NO/DATE: EU/1/15/1042 20150918 |
| 2114383 | CR 2016 00007 | Denmark | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL; REG. NO/DATE: EU/1/15/1042/001-006 20150922 |
| 2114383 | CA 2016 00007 | Denmark | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL, HERUNDER SUFENTANIL SOM CITRAT; REG. NO/DATE: EU/1/15/1042/001-006 20150922 |
| 2114383 | 300797 | Netherlands | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL, DESGEWENST IN DE VORM VAN SUFENTANILCITRAAT; REGISTRATION NO/DATE: EU/1/15/1042 20150922 |
| 2114383 | SPC/GB16/004 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL; REGISTERED: UK EU/1/15/1042 20150922 |
| 2114383 | 16C0010 | France | ⤷ Sign Up | PRODUCT NAME: SUFENTANIL; REGISTRATION NO/DATE: EU/1/15/1042 20150918 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


