Last updated: July 27, 2025
Introduction
Desoximetasone is a potent topical corticosteroid primarily prescribed for inflammatory and allergic skin conditions, including psoriasis, eczema, and dermatitis. Recognized for its anti-inflammatory, antipruritic, and vasoconstrictive properties, desoximetasone is marketed under various brand names globally. Understanding its market dynamics and financial trajectory entails analyzing clinical efficacy, regulatory landscape, competitive positioning, and evolving healthcare trends influencing its demand and revenue generation.
Pharmacological Profile and Therapeutic Indications
Desoximetasone acts by suppressing inflammatory cell migration and reversing capillary dilation, thereby reducing redness, swelling, and itching associated with dermatological conditions. Its high potency class positions it against other corticosteroids such as clobetasol propionate; however, its safety profile, especially risk of skin atrophy and systemic absorption, determines usage limitations [1].
Therapeutically, it chiefly targets moderate to severe inflammatory skin disorders. The drug's topical application offers localized relief with minimal systemic exposure, making it preferred in scenarios requiring stringent safety controls.
Market Size and Growth Drivers
The global dermatological therapeutics market, valued at approximately USD 23 billion in 2022, is projected to grow at a CAGR of around 6.2% through 2030 [2]. A key segment within this is corticosteroid-based medications, including desoximetasone.
Several catalysts underpin the market's upward trajectory:
-
Rising Prevalence of Skin Disorders: The increasing incidence of psoriasis, eczema, and contact dermatitis driven by lifestyle changes, pollution, and urbanization amplifies demand. Data indicates psoriasis affects over 125 million worldwide, with a rising trend in developing countries [3].
-
Advancement in Formulations: Innovations such as ultra-potent formulations, combined therapies, and novel delivery systems (e.g., foam, cream) elevate treatment adherence and efficacy.
-
Aging Population: Elderly demographics exhibit higher susceptibility to chronic dermatological conditions, stimulating sustained demand.
-
Growing Awareness and Healthcare Access: Enhanced awareness initiatives and expanding healthcare infrastructure, especially in emerging markets, facilitate broader prescription and over-the-counter availability.
Competitive Landscape
Desoximetasone faces competition from both other potent corticosteroids and alternative therapeutic classes. Market players include Novartis, Mylan, and Teva Pharmaceuticals, offering formulations under various brand names like Topicort (Lederle/AbbVie) and others.
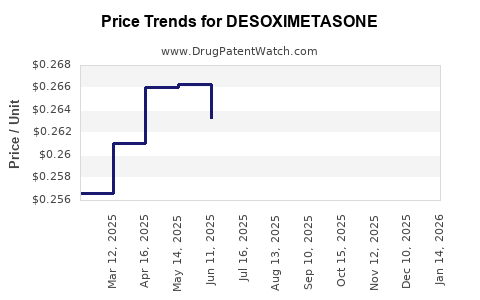
In addition to generic entries, the patent expiry of earlier corticosteroids has led to increased generic penetration, intensifying price competition. Notably, generic versions account for a significant market share, contributing to reduced pricing but also expanding patient accessibility [4].
Emerging biologic therapies targeting underlying immune pathways (e.g., IL-17 inhibitors for psoriasis) challenge corticosteroid dominance but remain primarily in the systemic or injectable category, giving topical corticosteroids a continued niche.
Regulatory Framework & Market Accessibility
Regulatory agencies such as the FDA, EMA, and other national bodies necessitate rigorous safety and efficacy data prior to approval. Desoximetasone’s high potency classification invites strict prescribing guidelines, with limitations on duration and application area to mitigate adverse effects.
The ease of over-the-counter availability varies globally, often influenced by safety considerations. While many markets restrict potent corticosteroids to prescriptions, expanding access in emerging economies remains a strategic focus, potentially increasing consumption volumes.
Financial Trajectory & Revenue Outlook
Financial projections for desoximetasone hinge on several factors:
-
Patent and Formulation Lifecycle: With patents having expired or nearing expiry, revenue streams face downward pressure due to generics. However, formulation innovations and new combination therapies offer pathways for revenue retention.
-
Market Penetration and Pricing Strategies: Companies leveraging aggressive pricing, targeted marketing, and expanding regional presence can offset volume declines and sustain margins.
-
Expanding Indications: Research exploring off-label uses or adjunct indications could open new markets, although such avenues require regulatory substantiation.
-
Regional Growth: Developing economies are expected to contribute proportionally to revenues owing to increasing dermatological condition prevalence and expanding healthcare access.
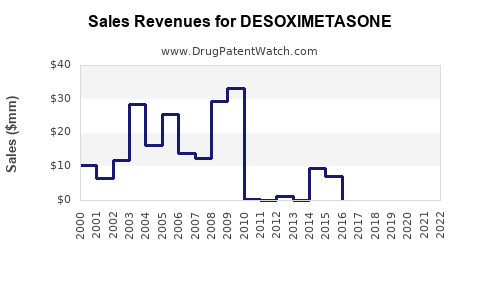
For example, a leading pharmaceutical company reported that topical corticosteroids, including desoximetasone, composed approximately 15% of its dermatology segment revenue in 2022. Given the market trends, a compounded annual decline of 2-3% is anticipated for branded desoximetasone products post-patent expiry, whereas generics could see a volume-driven increase.
Market Challenges and Risks
Despite positive growth prospects, several challenges threaten market stability:
-
Safety Concerns & Regulatory Restrictions: Adverse effects associated with potent corticosteroids, including skin thinning and systemic toxicity, lead to tighter use guidelines.
-
Pricing Pressures & Generics: Heightened competition from generics diminishes profit margins and presses manufacturers to innovate.
-
Emerging Alternatives: Biologics and non-steroid topical agents (e.g., calcineurin inhibitors) could supplant corticosteroids in certain indications, impacting long-term demand.
-
Regulatory Hurdles in Emerging Economies: Variations in approval processes/requirements can delay market entry or expansion plans.
Future Outlook & Strategic Considerations
The future landscape for desoximetasone is characterized by a shift toward tailored therapies and combination formulations. Companies are investing in:
-
Novel Delivery Systems: Improving drug stability, reducing adverse effects, and enhancing patient compliance.
-
Combination Therapies: Pairing corticosteroids with antimicrobials or moisturizers to extend indications and treatment efficacy.
-
Digital & Teledermatology: Leveraging remote consultation channels can increase prescription efficiency and patient adherence.
-
Market Expansion in Asia-Pacific and Latin America: These regions promise growth owing to rising disease prevalence and healthcare investments.
In the longer term, the transition from potent corticosteroids to safer, targeted therapies may influence desoximetasone’s market share. Nonetheless, its affordability and well-established efficacy ensure it remains a vital component in dermatological pharmacotherapy.
Key Takeaways
-
The demand for desoximetasone is driven by rising dermatological conditions, aging populations, and formulation innovations, anchoring a steady growth trajectory despite patent expiries.
-
Competitive forces, especially generic entries, exert downward pressure on revenues, compelling manufacturers to innovate in delivery and combination therapies.
-
Regulatory frameworks influence market accessibility, with stricter prescribing guidelines protecting patient safety but potentially limiting growth in some regions.
-
The expanding role of biologics and alternative agents presents both challenges and opportunities for desoximetasone’s positioning.
-
Strategic investments in formulation technology, regional expansion, and maintaining safety profiles are critical for sustaining profitability.
FAQs
1. What are the main therapeutic uses of desoximetasone?
Desoximetasone is primarily prescribed for inflammatory skin conditions such as psoriasis, eczema, and dermatitis, where it alleviates redness, swelling, and itching.
2. How does patent expiry impact desoximetasone's market?
Patent expiration often leads to increased generic competition, reducing prices and profit margins while expanding access. Companies may counter this with formulation innovations and new indications.
3. Are there safety concerns with using desoximetasone?
Yes. Potent corticosteroids like desoximetasone can cause skin atrophy, telangiectasia, and systemic effects if misused. Regulatory agencies impose strict prescribing guidelines to mitigate risks.
4. What emerging therapies could threaten desoximetasone's market share?
Biological agents targeting immune pathways (e.g., IL-17 inhibitors) and non-steroidal topical agents are emerging alternatives for certain conditions.
5. What regions offer the most growth opportunities for desoximetasone?
Emerging markets in Asia-Pacific, Latin America, and Africa are poised for growth due to increasing disease prevalence, improving healthcare infrastructure, and expanding medication access.
References
[1] Pharmacology and safety profile of topical corticosteroids. Journal of Dermatological Treatment. 2020.
[2] Global dermatology therapeutics market report. MarketResearch.com. 2022.
[3] Epidemiology of psoriasis worldwide. National Psoriasis Foundation. 2021.
[4] Impact of generic entry on corticosteroid markets. Pharmaceutical Market Analysis. 2022.