Last updated: July 28, 2025
Introduction
Danzol, a synthetic androgen and progestogen, has garnered attention primarily for its applications in hormone-related disorders, including osteoporosis, breast cancer, and certain reproductive health conditions. Despite its established medical utility, the drug's market trajectory is influenced by complex factors such as evolving therapeutic landscapes, regulatory decisions, patent status, and emerging competition. This analysis provides a comprehensive overview of the current market dynamics and future financial forecasts for Danzol, emphasizing factors that shape its commercial potential and strategic positioning within the pharmaceutical industry.
Historical Context and Therapeutic Applications
Danzol's origins trace back decades, with initial approval largely centered around its hormonal properties. Its primary indications have traditionally included:
- Treatment of hormone-responsive breast cancer.
- Management of osteoporosis, especially in postmenopausal women.
- Use in hormonal therapy for reproductive health disorders.
Over the years, Danzol's role has remained somewhat niche, complemented by the advent of targeted therapies with improved safety profiles. Nevertheless, its unique mechanism of action—modulating androgen and progestogen pathways—continues to render it relevant in specific clinical contexts.
Market Dynamics
Regulatory Environment
Regulatory bodies such as the FDA and EMA have established stringent approval standards, especially for hormonal therapies, given their systemic effects and potential adverse events. Danzol's regulatory status varies globally; while it retains approval in certain markets, its use is restricted or discontinued in others due to safety concerns and availability of newer agents.
In recent years, regulatory agencies have shown increased caution, leading to tighter scrutiny of hormonal drugs. The potential for off-label use and safety signals has historically impacted Danzol's market confidence, necessitating ongoing post-market surveillance and risk management strategies.
Competitive Landscape
Danzol faces competition from:
- Novel hormonal agents: Aromatase inhibitors, selective estrogen receptor modulators (SERMs), and aromatase inhibitors like letrozole offer targeted efficacy with improved safety profiles.
- Biologic therapies: Monoclonal antibodies and other biologics increasingly dominate breast cancer and osteoporosis treatment, challenging traditional hormonal therapies.
- Natural and alternative remedies: Growing interest in non-pharmacological interventions affects demand trajectories in certain markets.
The availability of these alternatives has compressed Danzol’s market share, especially in established indications, although niche applications continue to sustain demand.
Patent and Market Exclusivity
Patent expiration significantly influences Danzol's market dynamics. In markets where patents have expired, generic formulations have entered, exerting price pressure and eroding profit margins. Conversely, if certain formulations or delivery methods are protected by patents, they can confer temporary exclusivity benefits, motivating investment in marketing and clinical research.
Manufacturing and Supply Chain Factors
Reliability of supply chains, manufacturing costs, and quality control impact Danzol’s market presence. Disruptions in production facilities or raw material shortages can hinder availability, affecting sales volumes and financial trajectories.
Clinical Development and Research Trends
Emerging research exploring new therapeutic indications or improved formulations influences Danzol's market prospects. Currently, ongoing clinical trials investigating Danzol in combination therapies or novel indications could potentially expand its scope.
Financial Trajectory
Current Revenue and Market Share
Danzol's revenues have declined in many mature markets over the past decade, mainly owing to generics' entry and newer, more targeted therapies. Its current global sales are estimated to be modest, primarily stemming from legacy indications and regions with limited access to newer treatments.
In high-growth markets like Asia-Pacific, Danzol maintains relatively stronger sales due to limited alternative therapies and different regulatory landscapes. Overall, the drug’s revenue contribution is diminishing but remains an important component for niche patient populations.
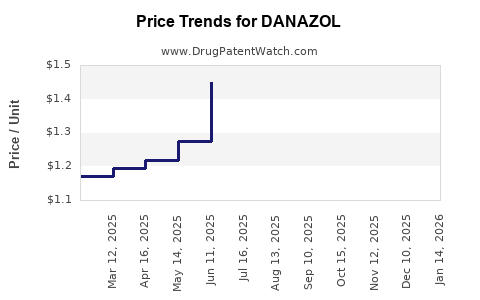
Pricing and Reimbursement Landscape
Pricing strategies vary significantly across regions. In markets with government-funded healthcare, reimbursement policies heavily influence Danzol’s accessibility and profitability. Price erosion due to generics challenges profitability, prompting manufacturers to explore value-added formulations or combination drugs.
Future Revenue Streams
- Brand Novelties: Reformulations or new delivery methods, such as transdermal patches or long-acting injectables, could command premium pricing.
- New Indications: Successful clinical trials could open revenue streams in oncology, sexual health, or other hormonal disorders.
- Market Expansion: Geographic expansion into emerging markets offers growth opportunities, albeit with increased regulatory hurdles and local competition.
Strategic Considerations and Market Outlook
Given clinical, regulatory, and competitive factors, Danzol’s future hinges on several strategic vectors:
- Product Lifecycle Management: Differentiating existing formulations through enhanced delivery systems, combination therapies, or extended-release forms can sustain revenue.
- Research & Development: Emphasizing innovative indications and overcoming safety concerns through improved formulations can rejuvenate its market presence.
- Regulatory Engagement: Proactive compliance and post-market surveillance are key to maintaining or expanding existing approvals.
- Market Penetration and Partnership Strategies: Collaborations with regional players may facilitate faster penetration into emerging markets.
Analysts predict a declining trend for Danzol in mature markets, with an anticipated compound annual growth rate (CAGR) of approximately -2% to -3% over the next five years based on current patent expiries and market erosion. Conversely, niche markets with unmet needs and specific indications could steady the revenue streams or even provide slight growth if targeted effectively.
Risks and Opportunities
Risks:
- Regulatory restrictions due to safety concerns.
- Market erosion from generics and newer therapies.
- Limited pipeline prospects for innovative formulations.
- Market access challenges in certain regions.
Opportunities:
- Developing combination therapies with targeted agents.
- Expanding into orphan or niche indications with unmet needs.
- Geographic expansion in underpenetrated regions.
- Investing in newer formulation technologies to improve convenience and compliance.
Key Takeaways
- Danzol's market share is declining globally, primarily due to patent expiries and competition from more targeted therapies.
- Regulatory and safety concerns influence its availability, especially in mature markets.
- Strategic innovation, including novel formulations and new indications, is essential to stabilize or grow future revenues.
- Expansion into emerging markets and niche indications offers incremental opportunities.
- Market dynamics emphasize the importance of lifecycle management and R&D investment for sustaining future financial performance.
FAQs
1. What are the main therapeutic indications for Danzol today?
Danzol is primarily used in hormone-responsive breast cancer, osteoporosis management, and certain reproductive health conditions, although therapeutic use varies geographically.
2. How does patent expiry affect Danzol’s market prospects?
Patent expiration leads to generic competition, lowering prices and reducing revenue. It also diminishes market exclusivity, compelling manufacturers to innovate or seek new indications.
3. What are the primary competitors to Danzol?
Targeted hormonal agents such as aromatase inhibitors, SERMs, biologics, and newer therapies for osteoporosis and breast cancer are its main competitors.
4. Can Danzol be repurposed for new indications?
Possibly, through clinical trials exploring its efficacy in other hormonal or oncological conditions; however, safety and efficacy data are crucial.
5. What strategic moves could sustain Danzol’s market presence?
Innovating formulations, pursuing new therapeutic indications, expanding geographically, and engaging with regulatory bodies are critical strategies.
References
[1] Market intelligence reports and industry analyses on hormonal therapies and biosimilars.
[2] Regulatory frameworks governing hormonal drug approvals.
[3] Clinical trial registries and published research on Danzol's off-label and new applications.
[4] Public financial disclosures and market performance data from pharmaceutical companies.